Adulteration of Ashwagandha
(Withania somnifera) Roots, and
Extracts
By Vineet Kumar Singh, MPharm,a Deepak Mundkinajeddu, PhD,a Amit Agarwal, PhD,a Jonathan Nguyen,b Sidney Sudberg,b Stefan Gafner, PhD,c Mark Blumenthalc
aNatural Remedies Private Limited, Bangalore, India
bAlkemist Labs, Garden Grove, CA 92841,
USA
cAmerican Botanical Council, Austin, TX 78723, USA
Correspondence: email
Citation
(JAMA style): Singh VK, Mundkinajeddu D, Agarwal A, Nguyen J, Sudberg S, Gafner
S, Blumenthal M. Adulteration of ashwagandha
(Withania somnifera) roots and extracts. Botanical Adulterants Prevention Bulletin. Austin, TX: ABC-AHP-NCNPR
Botanical Adulterants Prevention Program; 2018.
Keywords:
Adulterant,
adulteration, ashwagandha root, ashwagandha leaf, ashwagandha aerial part, Withania somnifera.
Goal: The goal of this bulletin is to
provide information and/or updates on the issue of adulteration of ashwagandha (Withania somnifera, Solanaceae) root materials
and their extracts to the international herbal products industry and extended
natural products community in general. It is intended to complement the
previously published work on W. somnifera
root and extract adulteration, i.e., the American Herbal Pharmacopoeia monograph
by Upton et al.1 and the article by Mundkinajeddu et al.2 by
reporting new data on the occurrence of adulteration, the market situation, and
its subsequent consequences on the industry and end users.
Scope: The focus of this bulletin is on the
sale of ashwagandha root powder and/or root extracts that contain undeclared
ashwagandha leaf and/or stem material for the unethical financial gain of the
seller. However, low amounts (i.e., below 2%) of leaf/aerial parts in roots or
root powder may be acceptable since these represent permissible contents of
foreign organic matter according to pharmacopeial standards.3 In
addition, ingredients and products containing mixtures of ashwagandha leaf and
root and/or their extracts, where the presence of both plant parts is appropriately
listed on the material’s certificate of analysis and/or finished product label,
are not considered adulteration and are not within the scope of this document.
1
General Information
1.1
Common name: Ashwagandha4
1.2 Other common names:
English: Indian ginseng*,5,6 winter
cherry7
Arabic: Bahman, ubad
Bengali: Ashvagandha, dhuppa
Chinese: Cui
mian shui qie (催眠睡茄), nan fei zui qie (南非醉茄)
Danish: Withania, blærebæger
French: Ashwagandha, cerise d’hiver,
coqueret somnifère, ginseng indien*
German: Ashwagandha, Indischer Ginseng*,
Schlafbeere, Winterkirsche
Gujarati: Ghodakun
Hindi: Asgandh
Italian: Ashwagandha, ciliegia d'inverno, ginseng indiano*
Kannada: Hiremaddina gadde
Malayalam: Amukkura
Marathi: Askandha
Nepalese: Aasoganda
Norwegian:
Withania, indisk
ginseng*
Persian:
Meheman
Punjabi: Asgand
Pustu: Kutilad
Sanskrit: Ashvagandha
Sinhalese: Amukkara
Spanish: Ashwagandha, cerezo de invierno,
ginseng indiano*, oroval
Swedish: Withania, indisk ginseng*
Tamil: Amukira, amukkara, asuragandi
Telugu: Panneru gaddu
Tibetan: Ba-dzi-gandha
*The ABC-AHP-NCNPR Botanical
Adulterants Prevention Program does not recommend the use of the inappropriate
common name “Indian ginseng” (or its translation into languages other than
English as noted above) in commercial trade or in the scientific or popular
literature. Ashwagandha has no botanical or chemical relationship or similarity
to plants that are appropriately referred to as “ginseng” in the herb trade
and/or in scientific and/or popular literature, i.e., plants from the genus Panax (family Araliaceae). The
inappropriate common names using the term ‘Indian ginseng’, or translations
into other languages where the term ‘ginseng’ is used, are provided simply as a
means of assisting quality control personnel et al. in identifying plant
material and/or extracts that contain W.
somnifera.
1.3
Latin binomial: Withania somnifera (L.) Dunal8
1.4
Synonyms: Physalis somnifera L.,
Withania kansuensis Kuang & A.M. Lu,
Withania microphysalis Suess8
1.5
Botanical family:
Solanaceae
1.6
Plant part and form: The
part used is the dried root, traditionally used as a powder. Much of the W. somnifera in the current market is
being supplied to herbal products and dietary supplement manufacturers in the
form of a dry extract. In most cases, the extract yield is approximately 10
times lower than the initial weight of raw material; i.e., 1 kg of dried root
yields 100 g of W. somnifera root
extract. The extract typically contains steroidal lactones called withanolides
in concentrations between 1.5-5.0% (w/w) in the extract.
1.7
General use(s):
In Ayurveda, ashwagandha is
claimed to have aphrodisiac, sedative, anxiolytic, rejuvenating, and life-prolonging
properties. According to The Ayurvedic
Pharmacopoeia of India, the
powdered dried root of ashwagandha is used to treat inflammatory disorders,
phthisis (any wasting or atrophic disease, weakness, diseases associated with vata
dosha [a body type, or
constitution in Ayurveda]), and male impotence.9 It is
one of the most important herbs of Ayurveda used for millennia as a Rasayana for its wide ranging health
benefits. Rasayana is described as a
preparation that promotes a youthful state of physical and mental health, and
expands happiness. These types of remedies are given to small children as
tonics, and are also taken by the middle-aged and elderly to increase longevity.
The Charaka Samhitaa, a Sanskrit text
on Ayurveda, classifies ashwagandha as Balya
(promoter of strength).10 It is also used as a general energy-increasing
tonic known as Medharasayana (promotes learning and memory function) and in geriatric
problems.11 The root of the plant has been traditionally used to
promote youthful vigor, endurance, strength, health, and increasing the
production of vital fluids, muscle fat, blood, lymph, semen, and cells.12
2
Market
2.1 Importance in the trade: In the US market, the vast majority of
ashwagandha supplements are sold in the Natural Channel. Sales in the two major
retail channels combined have steadily increased from an estimated US $4.53
million in 2014 to an estimated $12.24 million in 2017, corresponding to an
annual sales increase of ca. 39% (Table 1).
Table
1. Ashwagandha Dietary Supplement Sales in the US from 2012-2017
|
Channel
|
2014
|
2015
|
2016
|
2017
|
|
|
Rank
|
[US$]
|
Rank
|
[US$]
|
Rank
|
[US$]
|
Rank
|
[US$]
|
|
Naturala
|
22
|
3,765,921
|
12
|
5,722,569
|
8
|
8,732,489
|
6
|
10,625,382
|
|
MMOb,c
|
71
|
767,242
|
60
|
556,739
|
63
|
908,643
|
51
|
1,611,915
|
a According
to SPINS (SPINS does not track sales from Whole Foods Market.)
b According
to SPINS/IRI (The Mainstream Multi-Outlet [MMO] channel was formerly known as
the Food, Drug, and Mass Market channel [FDM], exclusive of possible sales at
Walmart, a major retailer in the United States and beyond.)
Sources:
T. Smith (American Botanical Council) e-mail to S. Gafner, September 2, 2015,
September 3, 2015, and June 19, 2018. K. Kawa (SPINS) e-mail to S. Gafner, July
11, 2016.
2.2 Market dynamics: While ashwagandha has a long-standing history of use in traditional
Indian medicine systems, interest in the benefits of ashwagandha roots and root
extracts in Australia, Europe, and North America has only recently started to
emerge. Sales of ashwagandha dietary supplements have seen double-digit growth
over the past years (Table 1). Current growth is said to be driven by the
increased awareness of benefits, such as stress relief and increase in energy, and
the increased support of benefits from published clinical studies.13
The increasing demand has created pressure for increased cultivation, which is
lagging behind the demand, according to a 2015 review article on conservation and
sustainability of the plant.14
2.3 Supply sources: Withania somnifera is
native to India and the Mediterranean region in North Africa, and it is widely
distributed in Pakistan, Sri Lanka, South Africa, Iraq, Iran, Syria, and
Turkey. In India, ashwagandha is commercially cultivated in Madhya Pradesh,
Gujarat, Maharashtra, Rajasthan, Haryana, Punjab, Karnataka, and Uttar Pradesh
provinces. In the Neemuch and Mandsaur districts of Madhya Pradesh province alone
the cultivated area exceeds 5000 hectares (ha) and, in India overall,
approximately 10,770 ha of land are used to grow ashwagandha with an annual
production of 8429 metric tons.14,15 In October 2018, costs for high
quality dried ashwagandha roots varied between US $2.46 – $3.56 on the Indian
market (although roots considered as lower grades, known as tar, were sold for as little as US $1.50),
compared to US $0.34 – 0.82 for dried ashwagandha leaves. (A. Agarwal personal
knowledge)
2.4
Raw material forms: Most
companies manufacture their own W.
somnifera root extract from dried roots, which is in agreement with the use
of ashwagandha in traditional Ayurvedic medicine. Consistent with this,
ashwagandha use is described primarily for its root in national pharmacopeias,
such as the United States Pharmacopeia (USP),3
the British
Pharmacopoeia,16
the Indian
Pharmacopoeia,17
the Ayurvedic
Pharmacopoeia,9
and reference works like the World Health Organization monograph.18 In
addition, a majority of the published clinical studies have been carried out
with ashwagandha root materials. Some proprietary ashwagandha ingredients appropriately
labeled to contain the extracts of aerial parts, leaves, or combinations of
roots and other plant parts are available on the North American market, and elsewhere.
Obviously, such materials, transparently and appropriately labeled, can be legally
marketed as ashwagandha supplements in the context discussed in this
publication.
3
Adulteration
3.1
Known adulterants: Undisclosed
non-root parts of W. somnifera, such as leaves, stem, and aerial parts of
ashwagandha, which are rich in withaferin A as well as other withanolides.
3.2
Information confirming adulteration: The quality evaluation of ashwagandha
raw materials and finished products has been the subject of three papers.2,19,20
Sangwan et al. found concentrations between 0.02 and 2.34 mg withaferin A per
gram of ashwagandha and highly variable chemical fingerprints in 10 commercial
products provided by dietary supplement manufacturers in India. The authors
commented that some of the results could be due in part to “unregulated and
often non-descript supplementation” of the root.19 Mundkinajeddu et
al. used high-performance liquid chromatography
with UV detection (HPLC-UV) to analyze authenticated samples of W. somnifera
leaves (n = 5), aerial parts (n = 3), and roots (n = 17), which were obtained
from India and Egypt.2 In
addition, 10 commercial extract samples labelled as "derived from the
roots" were analyzed for the presence of flavonol glycosides (Figure 1),
which are markers for adulteration with aerial parts. It was observed that only
two of the commercial extract samples did not contain any of the marker
compounds for aerial parts, indicating that aerial parts are sometimes used as
adulterants in ashwagandha root extracts. However, verification of 28 samples
of whole roots in the Indian state of Kerala did not find any evidence of
adulteration. The authors reported that occurrence of mold on the root surface
was a common problem.20 Data
on identity testing of 584 commercial raw material samples of ashwagandha root (Alkemist
Laboratories; Costa Mesa, CA) by high-performance thin-layer chromatography (HPTLC)
using the conditions outlined in Figure 2 showed that 119 samples (20.4%) were
not composed solely of authentic root material. Sample rejection was due to the
presence of leaf material in 84 samples (14.0%). (S. Sudberg [Alkemist
Laboratories], email communication to S. Gafner, May 12, 2017) Finally,
adulteration with ashwagandha leaf extracts was also mentioned by Shaheen Majeed
(Sabinsa Corporation, East Windsor, NJ) who commented that “Withania harvested roots mixed with
aerial parts and other plant parts have become a major concern.”13
3.3
Accidental or intentional adulteration: The motivation behind adulteration in commercial products
is financial gain. Since ashwagandha has seen a steady increase in sales, there
is more global demand for its roots. This has led to a considerable increase in
costs of roots, compared to the lower-cost aerial parts, which, as noted above,
also contain withanolides. Larger amounts of aerial parts can be collected in a
comparatively short time, which then can be made into extracts at a fraction of
the cost of producing root extracts and can be priced below the market rates of
authentic root extracts while providing a substantial profit for the
producer/seller. Accidental adulteration can happen at harvesting stage by the
farmers as some may not be aware of differences in the constituents and the
importance of using roots only rather than aerial parts. While cutting aerial
parts during the root harvesting process, they may cut the roots too far
aboveground, leading to raw materials that contain a small portion of aerial
parts.
3.4 Frequency of occurrence: The limited data available do not
provide clarity about the extent of adulteration. Ganzera et al. used HPLC-UV
to analyze six commercial products purchased in the United States. Two products
had higher contents of withaferin A, the compound found at high concentrations
in ashwagandha leaves, but the authors gave ”seasonal variations, a different
extraction procedure applied by the manufacturer or different chemotypes of the
plant” as possible reasons for the difference.21 Mundkinajeddu et
al.2 analyzed 10 commercial samples labeled as "derived from the roots" obtained from vendors and dietary ingredient
manufacturers from India for the
presence of flavonol glycosides (Figure 1) and concluded that eight products (80%) were found to be
adulterated with aerial parts. Another study, where
four herbal products (obtained from local stores in Coimbatore, India) labeled
to contain crude ashwagandha were evaluated using DNA barcoding (rbcL and ITS2 gene regions were
amplified), did not provide any evidence for adulteration with plant material
from a plant in a genus other than Withania.
Ashwagandha DNA sequences were obtained from three samples; the fourth sample
yielded only a short sequence using the rbcL
primer, and no sequence at all with ITS2.22 Since genetic methods
are unable to differentiate plant parts, the data from the DNA barcoding study
do not provide evidence that any of the four samples were made solely from ashwagandha root material. Based on the practical experience of
the authors, occurrence of accidental adulteration appears to be low.
3.5 Possible therapeutic issues: The use of Withania leaf extracts
appears to be safe. Data from studies with a product containing root and leaf
extracts showed mild and transient adverse events similar to those observed
with placebo.23,24
3.6
Analytical methods to detect adulteration: Documentary standards on ashwagandha have
been published by the American Herbal Pharmacopoeia,1 Ayurvedic
Pharmacopoeia of India,9 Siddha Pharmacopoeia of India,25
Unani Pharmacopoeia of India,26 the World Health Organization
(WHO) Monographs,18 as well as in the British
Pharmacopoeia,16 Indian Pharmacopoeia,17 and United
States Pharmacopeia.3
These standards cover microscopic, macroscopic, high-performance thin-layer chromatography
(HPTLC), and high-performance liquid chromatography (HPLC) methods for
identification of ashwagandha roots and quantification of withanolides. Parts of Withania somnifera other
than roots (e.g., stems, leaves) which have been used as adulterants, can be
identified when present in crude powdered form using microscopic analysis.27
An HPTLC method for
the detection of withanolides is listed also in the American Herbal Products
Association’s Botanical Identity References Compendium.28 Higher relative concentrations of withaferin A have been
reported in the leaves (typically >100 times than roots) and stems
(typically >10 times than roots).29 This could be used as an
indicator of the presence of undeclared aerial parts of W. somnifera in
roots. However, reliance on
withanolides as chemical markers to distinguish plant parts is hampered by the
occurrence of several ashwagandha chemotypes with differing withanolide
patterns.30,31 Therefore, Mundkinajeddu
et al. developed an HPLC method for simultaneous determination of the three
flavonoid glycosides quercetin 3-O-robinobioside-7-O-glucoside (1), quercetin 3-O-rutinoside-7-O-glucoside (2), and kaempferol 3-O-robinobioside-7-O-glucoside (3) which occur only in the aerial parts of W. somnifera.2
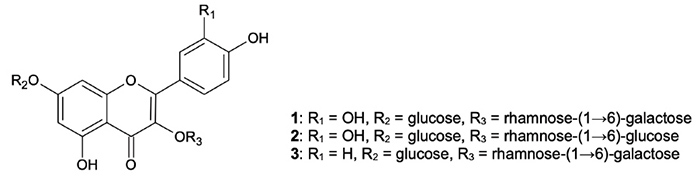
Figure
1: Principal flavonol glycosides in ashwagandha aerial parts
A limit test for flavonol glycosides has been included as an addition to the USP monographs on
Powdered Ashwagandha Root and Powdered Ashwagandha Root Extract, requiring
contents to be no more than 0.01% for powdered root, and 0.04% for extracts. A
clear distinction between leaf and root is also possible using 1H
NMR-based chemometric methods.32,33 The
two main metabolites that separate root samples from other plant parts in
50% aqueous methanol extracts were identified as
sucrose and γ-aminobutyric acid.31 DNA-based methods, such as those
reported by Shanmughanandhan et al.,22 are not appropriate
for the detection of root adulteration with various ashwagandha plant parts since there are no validated genetic
methods available to distinguish plant parts at this time.
Adulteration with leaf material can also be detected by HPTLC. Figure 2 shows
HPTLC profiles for authenticated W. somnifera root extracts, as well as
root extract samples adulterated with methanol and water extracts of the aerial
parts. Based on the data, admixture of as little as 5% methanol extracts of the
aerial parts can be detected by the presence of red bands (due to chlorophyll
pigments) at Rf = 0.2-35 and 0.9, but not for aqueous extracts of aerial parts.
The same samples were analyzed by HPLC (Figure 3) using the method by Mundkinajeddu et al.,2
where the presence of flavonol glycosides 1-3 in both the methanolic
extract and the water extract of the aerial parts was observed, thus confirming
the presence of the adulterant.
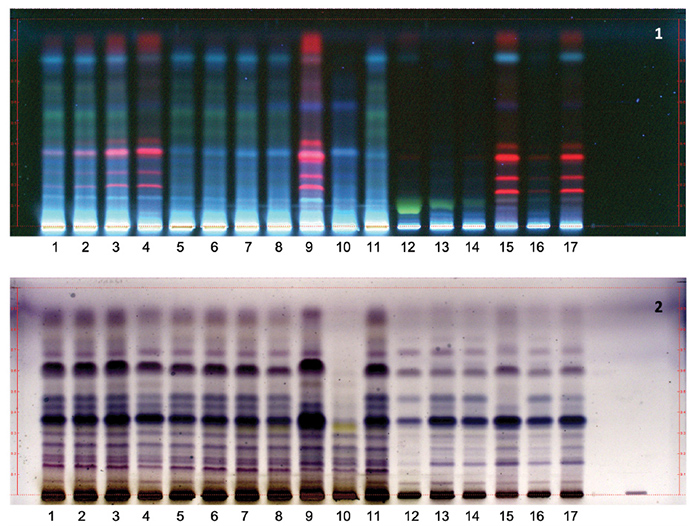
Figure 2. HPTLC analysis of ashwagandha root, aerial parts, and leaf
extracts, and mixtures of root and leaf extracts.
Lanes 1-4: 80% aqueous methanol
extracts of roots and aerial parts, mixed at 19:1, 9:1, 3:1, and 1:1 ratios,
respectively; lanes 5-8: 80% aqueous methanol extract of roots and water
extract of aerial parts, mixed at 19:1, 9:1, 3:1, and 1:1 ratios, respectively;
lane 9: 80% aqueous methanol extract of the aerial parts; lane 10: water
extract of the aerial parts; lanes 11-14: 80% aqueous methanol extracts of roots;
lane 15: 80% aqueous methanol extract of leaves; lanes 16 and 17: 80% aqueous methanol
extracts of roots and leaves, mixed at 19:1, and 1:1 ratios, respectively.
Stationary phase: Silica
gel 60, F254, 10 x 10 cm HPTLC plates.
Mobile phase: Methylene
chloride-methanol-acetone-diethyl ether [100:6.5:6.5:6.5] (v/v/v/v)
Detection: (1) UV at
365 nm
(2)
Vanillin/sulfuric acid reagent (after drying at 110oC for 5 min),
observed under white light
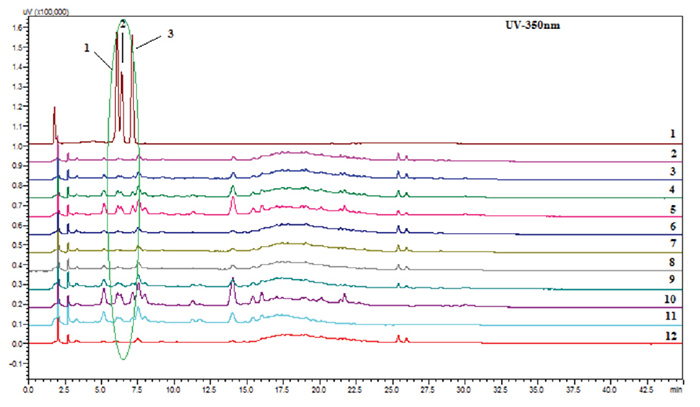
Figure 3: Representative HPLC at 350 nm chromatograms
of a standard mixture of quercetin 3-O-robinobioside-7-O-glucoside
(1), quercetin 3-O-rutinoside-7-O-glucoside (2), and kaempferol
3-O-robinobioside-7-O-glucoside (3, lane 1); mixtures of ashwagandha
80% aqueous methanol extracts of roots and aerial parts (lanes 2-5); mixtures of
ashwagandha 80% aqueous methanol extract of roots and water extract of aerial parts (lanes 6-9); 80% aqueous methanol
extract of the aerial parts
(lane 10); water extract of the aerial
parts (Lane 11); and 80% aqueous authentic root
extract (lane
12).
3.7
Perspectives: Withania somnifera extracts are often
“standardized” on the basis of their withanolide content, which are present in
aerial parts as well as roots. This has opened the possibility of undisclosed extracts
from aerial parts being used for intentional adulteration of W. somnifera root extract. There are quality dietary supplements where the addition of ashwagandha leaves, stems,
and aerial parts to W. somnifera root
extracts is appropriately labeled. However, the undeclared addition of various
plant parts other than the root with the sole intention of making a greater profit
for the seller is considered unethical and fraudulent. The expected increase in
the global demand for W. somnifera
root extracts in the coming years may further exacerbate pressures on the
supply chain and pricing, and hence, increase the risk of adulteration.
4
Conclusions
The adulteration of W. somnifera root extract by adding
undeclared extracts from aerial parts of the plant to commercial products
continues to provide potentially less value to the end user and impact the
reputation of the botanicals and natural products industry. This practice has a
considerable adverse impact on companies that sell genuine root material and extract,
because of the availability of lower-cost adulterated materials and extracts.
Validated analytical methods that enable detection of this type of adulteration
are available and should be adopted in every quality control laboratory. The
implementation of such validated methods by ethical suppliers and botanical
product manufacturers will provide appropriate testing data, as opposed to non-specific
spectrophotometric methods that often provide misleading results with regard to
the proper identity and authenticity of W.
somnifera root raw materials and extracts.
5
References
- Upton R, Petrone C, Swisher D.
American Herbal Pharmacopoeia: Ashwagandha Root, Withania somnifera: Analytical, Quality Control, and Therapeutic
Monograph. Scotts Valley, CA: American Herbal Pharmacopoeia, 2000.
- Mundkinajeddu
D, Sawant LP, Koshy R, et al. Development and validation of high performance
liquid chromatography method for simultaneous estimation of flavonoid
glycosides in Withania somnifera aerial
parts. ISRN Analytical Chemistry.
March 10, 2014;2014:351547. doi: 10.1155/2014/351547.
- United States Pharmacopeial
Convention. Ashwagandha Root; Powdered Ashwagandha Root; and Powdered
Ashwagandha Root Extract. In: United States Pharmacopoeia 42 and National
Formulary 37. Rockville, MD, USA: United States Pharmacopeial Convention, 2019;
4724-4730.
- Foster S, Awang D, Hu SY, Kartesz JT,
Tucker AO, Tyler VE. American Herbal
Products Association’s Herbs of Commerce. 1st ed. Silver Spring, MD:
American Herbal Products Association; 1992.
- Taxon: Withania somnifera (L.) Dunal. USDA Agricultural Research Service,
Germplasm Resources Information Network (ARS-GRIN). https://npgsweb.ars-grin.gov/gringlobal/taxonomydetail.aspx?id=102407. Accessed January 2, 2019.
- Wageningen, Gurib FA, Schmelzer GH. Withania somnifera (L.) Dunal. PROTA
(Plant Resources of Tropical Africa/Ressources Végétales de l’Afrique Tropicale).
www.prota.org (under construction as of 1/2/2019).
- Kapoor LD. CRC Handbook of Ayurvedic Medicinal Plants. Boca Raton, FL: CRC
Press; 1990.
- The Plant List. Version 1.1 (September
2013). Available at: http://www.theplantlist.org. Accessed October 2, 2016.
- Ayurvedic
Pharmacopoeia Committee. Asvagandha. In: The
Ayurvedic Pharmacopoeia of India, Part I, Volume I. New Delhi, India:
Government of India, Ministry of Health and Family Welfare, Department of
Ayurveda, Yoga & Naturopathy, Unani, Siddha and Homoeopathy (AYUSH), 1989.
- Dev S. Prime Ayurvedic Plant Drugs. New Dehli, India: Anamaya Publishers;
2006. CH SU-IV/9
- Nadkarni KM Indian Materia Medica. Bombay, India: Popular Prakshan Limited,
1976; 1291.
- Williamson EM. Major Herbs of Ayurveda. London: Churchill Livingstone, 2002;322-323.
- Daniells S. An herb to watch:
ashwagandha science growing consumer recognition and sales. NutraIngredients-USA
website. Available at: http://www.nutraingredients-usa.com/Markets/An-herb-to-watch-Ashwagandha-science-growing-consumer-recognition-and-sales. Published March 30, 2016. Updated
April 1, 2016. Accessed January 17, 2017.
- Shinde A, Gahunge P, Rath SK. Conservation
and sustainability of Ashwagandha: A medicinal plant. J Biol Sci Opin. 2015;3(2):94-99.
- Shrivastava AK, Sahu PK. Economics of yield and production of alkaloid of Withania somnifera (L.) Dunal. Am J Plant Sci. 2013;4:2023-2030.
- British
Pharmacopoeia Commission. Withania
somnifera for use in THMP. In: British
Pharmacopoeia 2012. London: The Stationery Office on behalf of the
Medicines and Healthcare products Regulatory Agency (MHRA). 2012.
- Government
of India Ministry of Health and Family Welfare. Indian Pharmacopoeia 2010. Ghaziabad, India: The Indian
Pharmacopoeia Commission; 2010.
- World
Health Organization. Radix Withaniae. In: WHO
Monographs on Selected Medicinal Plants. Volume 4. Geneva, Switzerland:
World Health Organization, 2009;373-391.
- Sangwan RS, Chaurasiya ND, Misra LN,
et al. Phytochemical variability in commercial herbal products and preparations
of Withania somnifera (ashwagandha). Curr Sci. 2004;86(3):461-465.
- Shalini R, Eapen JK, Deepa MS.
Macroscopic evaluation of genuine and market samples of ashwagandha (Withania
somnifera (Linn.) Dunal) in Kerala. J Pharmacogn
Phytochem. 2017:6(6);2283-2288.
- Ganzera M, Choudhary, Khan IA.
Quantitative HPLC analysis of withanolides in Withania somnifera. Fitoterapia.
2003;74(1-2):68-76.
- Shanmughanandhan D, Ragupathy S,
Newmaster SG, Mohanasundaram S, Sathishkumar R. Estimating herbal product
authentication and adulteration in India using a vouchered, DNA-based
biological reference material library. Drug
Safety. 2016;39(12):1211-1227.
- Chengappa KNR, Bowie
CR, Schlicht PJ, Fleet D, Brar JS, Jindal R. Randomized placebo-controlled
adjunctive study of an extract of Withania
somnifera for cognitive dysfunction in bipolar disorder. J Clin
Psychiatry. 2013;74(11):1076-1083.
- Ramakanth GSH, Uday Kumar C,
Kishan PV, Usharani P. A randomized, double blind placebo controlled study of efficacy and tolerability of Withania somnifera extracts in knee
joint pain. J Ayurveda Integr Med. 2016;7(3):151-157.
- Siddha Pharmacopoeia
Committee. Siddha Pharmacopoeia of
India, Part I, Vol. I, First Edition. New Delhi, India: Govt. of India,
Department of Ayurveda, Yoga & Naturopathy, Unani, Siddha and Homoeopathy
(AYUSH), 2008
- Unani Pharmacopoeia
Committee. The Unani Pharmacopoeia
of India, Part I, Volume I, New Delhi, India: Government of India, Ministry
of Health and Family Welfare, Department of Ayurveda,
Yoga & Naturopathy, Unani, Siddha and Homoeopathy (AYUSH). 2007;7-8.
- Atal CK, Gupta OP, Raghunanthan K, et
al. Pharmacognosy and phytochemistry of Withania
somnifera (Linn.) Dunal (ashwagandha). New Delhi, India: Central Council for Research in
Indian Medicine and Homoeopathy, 1975;47-53.
- American Herbal Products Association. Withania somnifera (root). AHPA
Botanical Identity References Compendium. http://www.botanicalauthentication.org/index.php/Withania_somnifera_(root). Accessed January 19, 2017.
- Chandra P, Kannujia R, Saxena A, et
al. Quantitative determination of multi markers in five varieties of Withania somnifera using ultra-high
performance liquid chromatography with hybrid triple quadrupole linear ion trap
mass spectrometer combined with multivariate analysis: Application to pharmaceutical
dosage forms. J Pharm Biomed Anal.
2016;129: 419–426.
- Kaul MK, Kumar A, Ahuja A, Mir BA, Suri KA, Qazi GN.
Production dynamics of withaferin
A in Withania somnifera (L.)
Dunal complex. Nat Prod Res. 2009;23(14):1304-1311.
- Kushwaha S, Roy S,
Maity R, et al. Chemotypical variations in Withania somnifera lead to differentially
modulated immune response
in BALB/c mice. Vaccine. 2012;
30(6):1083-1093.
- Namdeo AG, Sharma A, Yadav KN, et al. Metabolic characterization of Withania somnifera from different regions of India using NMR spectroscopy. Planta Med. 2011;77(17):1958-1964.
- Chatterjee S, Srivastava S, Khalid A, et al. Comprehensive
metabolic fingerprinting of Withania
somnifera leaf and root extracts. Phytochemistry.
2010;71(10):1085-1094.
|
Version # , Author
|
Date Revised
|
Section Revised
|
List of Changes
|
|
Version 1,
V.K. Singh, D. Mundkinajeddu, A. Agarwal, J. Nguyen, S. Sudberg, S. Gafner,
M. Blumenthal
|
n/a
|
n/a
|
none
|