 Click to
view or download PDF version
Click to
view or download PDF version
Boswellia serrata Adulteration
By
Allison McCutcheon, PhD
American Botanical
Council, PO Box 144345, Austin, TX 78714
Correspondence: e-mail
Citation (JAMA
style): McCutcheon A. Adulteration of Boswellia serrata. Austin, TX:
Botanical Adulterants Prevention Program; Botanical Adulterants Bulletin. 2018.
Keywords: Adulterant,
adulteration, Boswellia, Boswellia
serrata, Boswellia carteri, Boswellia frereana, Boswellia
sacra, Boswellia papyrifera boswellic acids, Garuga pinnata, Indian frankincense, Pinaceae
Goal:
The goal of this bulletin is to provide timely information and/or updates on
issues of adulteration of Boswellia serrata
(Burseraceae) to the international herbal industry and extended natural
products community in general. It is intended to complement the previously
published works with information on B.
serrata adulteration by presenting new data on the occurrence of
adulteration, the market situation, and consequences for the consumer and the
industry.
1 General Information
1.1 Common name: Indian frankincense,1 boswellia2
1.2 Other common names:
English:
Indian olibanum2,3
Assamese: Sallaki4
Ayurvedic: Shallaki, susravaa,
gajabhakshyaa, salai, gum-kunduru5
Bemba: Kundru6
Bengali: Luban, salai,6 salgai7
Chinese: Chi
ye ru xiang shu (齿叶乳香树)
French: Arbre à encens de l'Inde, boswellie, encens d’Inde2
Gujarati: Gugal, saleda, dhup,6 shaledum,
saladi, gugal, saledhi4
German: Indischer
Weihrauch2
Hindi: Madi, salai, saler, salga, salhe,
sali,6 anduk, gugal, halar, kundur, loban, lobhan,
luban, salaga, salai, salar, salaran, salhe, sel-gond, vellakkunturukkam,7
labana4
Italian: Incenso
indiano8
Kannada:
Madimar, chilakdupa, tallaki, maddi4
Kashmiri:
Kunturukkam, samprani4
Marathi: Salai
cha dink4
Pharmacopoeial:
Olibanum Indicum,9 Gummi Boswellii10
Punjabi:
Salaigonda4
Sanskrit: Sallaki, kunduru,6 agavrttika,
ashvamutri, asraphala, bahusrava, gajabhaksha, gajabhaksya, gajapriya,
gajasana, gajashana, gajavallabha, gandhamula, gandhavira, guggu, hladini,
hraswada, jalatiktika, kapitthaparni, karaka, khapurah, konkanadhoopam,
konkanadhupa, kumbhi, [kundara, kundu, kundurakam, kunduru, kunduruguggulu,
kunduruka, kunduruki, kunduruska, kunduruskah; see comments by Dymock et al.11],
lhadini, maherana, maheruna, mocha, nagavadhu, nagavrttika, rasala, salakhi,
salasi, salasiniryasam, salasiniryasasallaki, sallaki, shallaki, silhabhumika,
silhaki, sugandha, sukhamoda, surabhi, surabhisrava, sushrika, susrava, suvaha,
vanakarnika, vasamaharuba, viseshadhupa, vrttika, yakshadhupa7
Sri
Lankan: Kundirikkan12
Spanish: Incienso indio13
Tamil: Parangisambrani, kungli, kundrikam,
gugulu, morada,6 kundurukam4
Telugu: Anduga, kondagugitamu4
Trade
names: Salai, kundur
luban,2 lobhan, salakhi,3 dupa, guggul, kaadar, salai
guggul7
Unani: Kundur5
Urdu: Kundar,4
kundur, loban, lobana, sat loban7
1.3 Accepted Latin binomial: Boswellia
serrata Roxb. ex Colebr.14,15
1.4
Synonyms: Boswellia balsamifera Spreng., Boswellia glabra Roxb., Boswellia thurifera Roxb. ex Fleming,
Chloroxylon dupada Buch.-Ham., Libanotus asiaticus Stackh.,3,14
Libanus thuriferus Colebr.15
1.5 Botanical family: Burseraceae
1.6
Botanical taxonomy:
The genus Boswellia consists of 28
species of trees and shrubs.14 In addition to B. serrata, the species Boswellia
frereana Birdw. (elemi frankincense), Boswellia
papyrifera (Caill. ex Delile) Hochst. (elephant tree), and Boswellia sacra Flueck., syn. B. carteri Birdw. (frankincense, olibanum)
are also commercially important species; all four species are traded under the
common name frankincense.16,17
Note:
Throughout this
document, the currently accepted scientific names are used and the older
synonyms used in the literature cited are noted in parenthesis. In the
literature, B. carteri is sometimes
spelled B. carterii; the former
spelling is correct and is used throughout this document.14-16
1.7
Distribution range:
Dry, rocky ridges and slopes, as well as flat terrain in Bangladesh, India,
Pakistan, and Sri Lanka.12,15 In general, the distribution of B. serrata in India includes the Indian
Punjab, Uttar Pradesh, Madhya Pradesh, Rajasthan, Maharashtra, Andhra Pradesh,
Karnataka, and Tamil Nadu.18 More specifically, B. serrata is found in the following Indian states: Karnataka, Kerala,
Tamil Nadu, Andhra Pradesh, Chattisgarh, Orissa, West Bengal, and Maharashtra.19
Boswellia frereana is native to
Somalia; it has also been reported in Ethiopia and Yemen.20
Boswellia papyrifera is
indigenous to Cameroon, Chad, Ethiopia, Eritrea, Nigeria, Sudan, and Uganda.21,22
Boswellia sacra (syn. B. carteri) has a disjunct distribution
in Arabia (Oman and Yemen; B. sacra
phenotype) and Somalia (B. carteri
phenotype).23,24
1.8 Plant part, form, and production method: Air-dried oleogum resin exudate
from the tree bark. In Ayurvedic medicine, the dried resin is most commonly used,
while in the American and European markets, B.
serrata is almost exclusively sold in the form of resin extracts. Extracts
may be standardized based on the content of total acids, organic acids,
boswellic acids (BA; 60–70%), 3-O-acetyl-11-keto
boswellic acid (AKBA; 30%), or 3-O-acetyl-β-boswellic
acid (AβBA; 20%).25
1.9
General use(s): The management of chronic inflammatory conditions including arthritis,
bronchial asthma, Crohn's disease, rheumatoid arthritis, and ulcerative
colitis.4,10
2
Market
2.1 Importance in the trade: From 2013 to 2016, retail sales of B. serrata dietary supplements in the
Natural channel in the United States (US) have shown a steady growth, with an
average annual sales increase of 11.1%. Sales growth in the Mainstream
Multi-Outlet channel from 2013 to 2016 was impressive, where B. serrata dietary supplements showed
one of the highest growth rates (413.3% annual average) for any botanical in
the United States.
Table
1. Boswellia Dietary Supplement Sales in the US from 2012–2016
|
Channel
|
2013
|
2014
|
2015
|
2016
|
|
|
Rank
|
Sales
[US$]
|
Rank
|
Sales
[US$]
|
Rank
|
Sales
[US$]
|
Rank
|
Sales
[US$]
|
|
Naturala
|
53
|
1,546,219
|
48
|
1,695,605
|
42
|
1,884,443
|
45
|
2,122,150
|
|
Mainstream Multi-Outletb
|
114
|
142,923
|
70
|
770,654
|
33
|
5,966,583
|
22
|
13,341,744
|
a According to SPINS (SPINS does not track sales from Whole Foods Market.)
b Data from 2013–2015 according to SPINS/IRI. This channel includes the food, drug, and mass-market sector, military commissaries, and select buyer’s clubs and so-called dollar stores. SPINS/IRI data does not include discount department store sales, e.g., possible sales at Walmart and club stores are excluded, or products sold through the internet or health care practitioners.
Sources: Smith et al.26 T. Smith (American Botanical Council) e-mail to S. Gafner, September 2, 2015, September 3, 2015, and February 6, 2018. K. Kawa (SPINS) e-mail to S. Gafner, July 11, 2016.
On
the supply side, a price range of 100-300 Indian rupees per kilogram (US$1.47-4.41)
boswellia oleogum was listed in a paper on the trade of Indian medicinal plants,
co-published in 2017 by the National Medicinal Plants Board and the Indian
Council of Forestry Research & Education.27
2.2 Supply sources:
According
to the Indian Medicinal Plants Database (IMPD),7 B. serrata oleogum resin is wildcrafted
from dry tropical forests in most Indian provinces, except Assam and Bengal.
IMPD reported 500–1000 metric tons was harvested in 2008.7 The same harvest
volume was given in a 2017 publication by the Indian government.27
2
Adulteration
2.1
Known adulterants and substituents: Boswellia frereana, B. sacra (syn. B. carteri), other
Boswellia species, Garuga pinnata
(Burseraceae), Pinaceae resin (resin from tree species in the family Pinaceae).
Due to boswellia oleogum’s becoming locally scarce, instances of replacement of the
tree oleogum with bark or with soil collected near the tree has been reported (G.
Ravikanth [Ashoka Trust for Research in Ecology and the Environment] email to
S. Gafner, April 17, 2018)
While
B. serrata is the only species used
in traditional Indian herbal medicine systems (Ayurveda, Siddha, and Unani),
the Pharmacopoeia of the People’s
Republic of China allows the interchangeable use of B. sacra with gums of closely related trees from the same genus.28
Similarly, B. serrata and B. sacra are both used for the same
indications in systems of Islamic herbal medicine, although depending on the
region, only the locally present Boswellia
species may be used.29,30
3.2 Sources of information confirming adulteration: Meins et al.25 assessed
the boswellic acid (BA) content of 17 top-selling American and European Boswellia products using liquid
chromatography-mass spectrometry (LC-MS). The specific products were selected
based upon data obtained from SPINS for the American market and from IMS OTC for
the European market (52-week sales ending December 2014). Six products
representing 78% of the units sold and 70% of the market share in the US were
purchased, four from retail stores and two over the internet. Eleven European
products representing 30% of the units sold and 40% of the market value were
purchased from German pharmacies. All of the product labels specified the
content was B. serrata extract except
for one which was labeled "Boswellia
extract." One product from Italy did not contain any of the six BAs (see
Figure 1) characteristic of B. serrata
(and B. sacra) and another product
from the United States contained only trace amounts, "suggesting the
absence of B. serrata or the use of
another Boswellia species such as B. frereana." Another product had
non-acetylated to acetylated BA ratios that were <1, indicating the presence
of B. sacra,* not B. serrata (see
section 3.7). In addition, two products did not meet their label claim for BA
content and another two products did not declare the use of extracts enriched
in AKBA.
Niebler and
Buettner24 conducted a comparative analysis of 46 commercial samples
of four Boswellia species using headspace (HS)–solid
phase microextraction (SPME) gas
chromatography–mass spectrometry (GC-MS).
Of the seven B. serrata oleogum resin
products that were analyzed, three exhibited abnormalities in their chemical
profiles. One showed the typical B.
serrata profile but had two additional unidentified peaks not found in any
other B. serrata sample and "a
noteworthy peak of β-longipinene
(1.4%) was detected, which was otherwise only detected in one other sample from
B. serrata." Two products contained more
than 10 peaks not commonly found in B.
serrata; longifolene (34.3% and 19.0% of the total peak area) was the highest peak in both
chromatograms and α-longipinene (1.7% and 2.1%), longicyclene (2.9% and
2.8%), longicamphylenone (0.44% and not detected), longiborneol (0.46% and
0.26%), and longiborneol acetate (3.0% and 2.3%) were also tentatively
identified. These
longifolene- and longipinene-type sesquiterpenes are characteristic of Pinaceae
resins and it is reported that a sample of Norway spruce (Picea abies, Pinaceae) resin produced a similar but not identical
chromatogram. The authors hypothesize that these two products may have been
adulterated with a Pinaceae resin. Among the samples from the other three Boswellia species, another six products
were found to contain either a mixture of Boswellia
species or did not contain the species stated on the label.
As a number
of authors have discussed,17,22,24,31-33 the scientific literature
on the chemical composition of commercial Boswellia
species contains many conflicting reports, some of which may be due to the use
of adulterated or misidentified commercial samples as the research material.
For example, Niebler and Buettner24 cite nine other papers which
concur with their analysis of authenticated Boswellia
references samples17,22,31,34-39 and note the following:
- Barratta et al.40 analyzed material purported to be B. serrata (syn B. thurifera); however, the chemical profile they presented resembles that of B. sacra. "The same disagreement can be found in the study by Van Vuuren et al.,41 if B. thurifera indeed refers to B. serrata."
- Kasali et al.42 analyzed B. serrata material purchased from markets in Nigeria; however, the results resemble the profile of B. rivae or B. neglecta. Niebler and Buettner also point out that "the only species native to Nigeria is B. dalzielii, which would better account for its availability on Nigerian markets."24
- Singh et al.43 reported a notably different chemical composition for B. serrata with major peaks for the compounds tetrahydrolinalool, benzyl tiglate, and methylisoeugenol. Niebler and Buettner could not confirm these results.24
- Six studies have reported octyl acetate or incensole acetate as major constituents of B. sacra (syn B. carteri).44-49 However, Niebler and Buettner did not find appreciable amounts of these compounds in authentic B. sacra samples; they note that the chemical profile reported in these articles is characteristic of B. papyrifera.24
- Hayashi et al.46 identified samples from Oman as B. frereana and those from Israel and Turkey as B. carteri; however, the data of Niebler and Buettner suggests that the Omani material was B. sacra and the Israeli and Turkish material was B. frereana.24
- In the case of B. frereana, Niebler and Buettner could not confirm the presence of notable amounts of β-caryophyllene reported by Van Vuuren41 in two out of three samples and they note that β-caryophyllene is a major component of B. sacra.24
Shanmughanandhan
et al.50 used the DNA barcode regions rbcL and ITS2 and a DNA
reference library for 187 species on Indian herbs to assess the botanical
authenticity and potential adulteration of 93 retail herbal products purchased
in Coimbatore, India. They reported that "60% were adulterated (i.e., herbal
products contained species of plants not listed on the label). Product
contamination was [reportedly] evident in 50% of the samples, while 10% of the
samples were substituted and 6% of the products contained fillers." The authors tested only one B. serrata product, which was found to
be adulterated with DNA from a Lamiaceae species (rbcL) and Trigonella foenum-graecum
(Fabaceae) (ITS2). [It should be
noted that the reliability of this analytical approach has been questioned due
to obvious methodological flaws which made the results prone to error,51
and the veracity of this data appears even more uncertain if the DNA reference material
used was the library of 187 Western herbs created by Newmaster et al.52]
Mishra et
al.53† reported Garuga pinnata
(Burseraceae) and B. sacra (syn B. carteri) as adulterants of B. serrata. The Ayurvedic Drugs website54
mentions the adulteration of B. serrata with moina gum from Garuga
pinnata, as well as adulteration with B. sacra and B. frereana "imported from countries of the Gulf and North Africa,
sold in the Indian market by the name Kundur." However, these
sources do not indicate how these adulterants were identified, nor the extent
to which such adulteration may occur. Moina gum is also listed as an adulterant
in a training manual on medicinal plant identification issued by the Andra
Pradesh State Forest Department.55
3.3 Accidental or intentional adulteration: As B. frereana and B. sacra
do not occur in the same geographical region as B. serrata, their presence in B.
serrata products must be due to either substitution based on interchangeable
use in certain cultures (see 3.1), misidentification, or intentional
adulteration at some point in the supply chain. The latter two ultimately are
indicative of poor quality control. Further, it is difficult to construe the
occurrence of pine resin as accidental adulteration.
3.4 Frequency of occurrence: There are no published studies on
the frequency of B. serrata
adulteration. Meins et al.25 found 3/17 (18%) and Niebler and
Buettner24 reported 3/7 (43%) of the commercial products that they assessed
were adulterated. The numerous conflicting reports on the chemical composition
of commercial Boswellia species in
the scientific literature also provides evidence that Boswellia adulteration commonly occurs when researchers use
material purchased from the commercial market.
3.5 Possible safety/therapeutic issues: The possible safety issues arising from the
substitution of B. serrata with B.
frereana, B. sacra, or
pine resin have not
been evaluated although all three have a history of safe traditional use as
medicines. The potential impact on therapeutic efficacy remains unknown;
however, it may be hypothesized that the substitution of B.
frereana which does
not contain the bioactive BAs of B. serrata may result in an ineffective
product. While B. sacra contains the six BAs characteristic of B. serrata, they are present in different
proportions and supporting clinical evidence of B. sacra anti-inflammatory efficacy is lacking.
3.6 Pharmacopeial standards: The United States Pharmacopeia (USP)
defines B. serrata as the oleogum
resin obtained by incision or produced by spontaneous exudation from the stem
and branches and specifies that it must contain not less than 1% of the keto
derivatives of β-boswellic acids, calculated on the dried basis as the sum of
11-keto-β-boswellic acid (KBA) and AKBA.56 The European
Pharmacopoeia (PhEur) specifies a minimum content of 1% KBA and 1% AKBA.57
3.7 Analytical methods to detect adulteration: Bioactivity researchers have largely
focused on six boswellic acids that are characteristic to B. serrata (see Figure 1), α-boswellic acid (αBA), 3-O-acetyl-α-boswellic acid (AαBA), β-boswellic
acid (βBA), 3-O-acetyl-β-boswellic
acid (AβBA), AKBA, and KBA. Studies have shown that these six BAs are present
in both B. serrata and B. sacra but are absent in B. frereana.58,59 Boswellia serrata can be distinguished
from B. sacra based on the ratios of
αBA/AαBA and βBA/AβBA which are 0.5-0.9 and 0.7-0.8 respectively in B. sacra and 1.7 and 1.2 in B. serrata. However, these ratios were
obtained with a small number of samples and need to be confirmed in a much
larger sample set. According to Frank and Unger, B. sacra also exhibits much lower signal intensities of KBA and AKBA
compared to B. serrata.58 Contrarily,
it has also been reported that the total BA and AKBA content is higher in B. sacra (49% and 7%, respectively)
extract compared B. serrata which
contains 30% and 0.7%, respectively.60
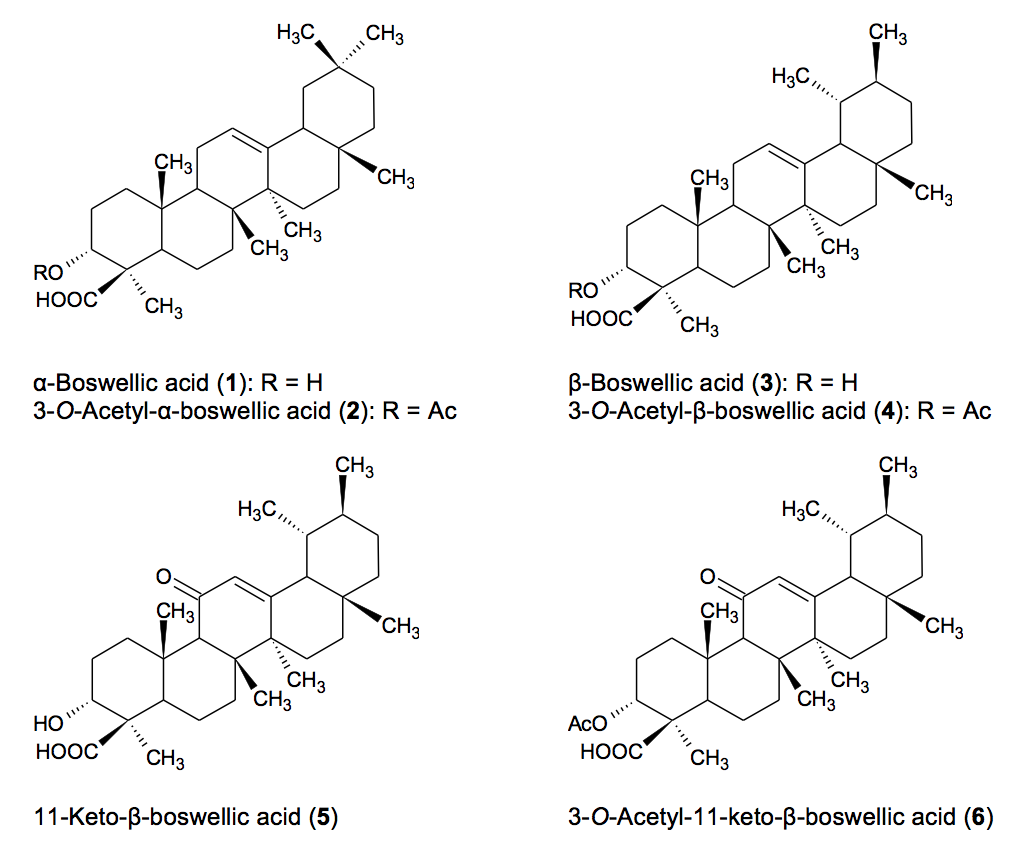
Figure 1: Structures of the principal boswellic acids in Boswellia serrata
Both the USP and PhEur specify the use of thin-layer chromatography (TLC) for identification and high-performance liquid chromatography (HPLC) for the quantification of βBA.56,57 Paul et al. described a TLC method that allows for the reliable discrimination of B. papyrifera, B. sacra, and B. serrata.17 Liquid chromatography mass spectrometry58 and gas chromatograph mass spectrometry24 methods for differentiating Boswellia species have also been published, along with suggested marker compounds for B. frereana, B. papyrifera, B. sacra, and B. serrata.22,24,31,36
3.8 Perspectives: The commercial supply of B. serrata is sourced almost exclusively from India. Therefore, country of origin provides an important indicator of the potential authenticity of purported B. serrata resins. In particular, the botanical identity of material originating from Africa, Arabia, and China should be rigorously verified.
Both B. papyrifera and B. sacra (and possibly other "non-commercial" species of Boswellia) also contain BAs and similarly exhibit analgesic properties. Therefore, quality assurance protocols limited to the quantification of BA content by HPLC may not detect adulteration with these species. And companies that quantify only total acids or total organic acids are at even greater risk of accepting adulterated material. This issue is simply addressed by employing the TLC method for the differentiation of B. papyrifera, B. sacra, and B. serrata described by Paul et al. as a preliminary screening.17
Over the past decade, the sales of B. serrata have continued to grow in proportion with the increasing body of evidence supporting its clinical efficacy. Perhaps the single greatest threat to the continued expansion of this sector is the presence of adulterated, ineffective products in the marketplace.
4 Conclusions
Based on the available data, it appears that the adulteration of B. serrata resin is both a common and long-standing occurrence that must be addressed with appropriate quality control protocols. Lack of efficacy due to adulteration with other Boswellia species and/or other lower cost resins has the potential to damage or even destroy consumer confidence in B. serrata products.
When ordering B. serrata from countries where Boswellia species can be used interchangeably, e.g., from China, manufacturers that specify a particular species on their product label should be aware of such interchangeable use and implement adequate quality control measurements to ensure that the purchased materials comply with the identity specifications for the desired species.
*Meins et al.25 refer to the species B. sacra and B. carteri as separate entities; however, B. carteri is a synonym for B. sacra.
†Mishra et al. cite the source of this
information as Alam MZ. Herbal Medicines. New Delhi: APH Publishing Corporation, 2008
(not accessed).
5 References
- McGuffin M, Leung A, Tucker
AO. Herbs of Commerce. Silver Spring,
MD: American Herbal Products Association. 2001.
- United
States National Plant Germplasm System online database. Available at: https://www.ars-grin.gov/misc/mmpnd/Boswellia.html.
Accessed August 12, 2016.
- Global
Biodiversity Information Facility (GBIF) online database. Available at: http://www.gbif.org/species/5421354/vernaculars.
Accessed October 8, 2016.
- The Ayurvedic Pharmacopoeia of India, Part I, Vol.
IV. New Delhi, Ministry of Health and Family Welfare, Department of Indian
System of Medicine and Homeopathy, 1999.Available
at: http://ayush.gov.in/sites/default/files/Ayurvedic%20Pharmacopoeia%20of%20India%20part%201%20volume%20IX.pdf.
Accessed June 2, 2017.
- Khare CP, ed. Indian Medicinal Plants: An Illustrated Dictionary.
New York: Springer. 2007. Available at: http://www.iauamol.ac.ir/Files/medicinal%20plants.pdf.
Accessed June 2, 2017.
- Orwa C,
Mutua A, Kindt R, Jamnadass R, Anthony S. 2009 Agroforestree Database: a tree
reference and selection guide version 4.0. World Agroforestry Centre (WAC). Available at: http://www.worldagroforestry.org/treedb/AFTPDFS/Boswellia_serrata.pdf.
Accessed October 14, 2016.
- Indian
Medicinal Plants Database: Trade. Available at: http://www.medicinalplants.in/trade.
Accessed June 2, 2017.
- Lawless J. Enciclopedia degli oli essentiali. Milan, Italy; Tecniche Nuove.
1992.
- European Scientific
Cooperative on Phytotherapy. E/S/C/O/P
Monographs: The Scientific Foundation for Herbal Medicinal Products. Second
Edition, Supplement 2009.Stuttgart; Thieme, 2009.
- Gummi Boswellii. WHO Monographs on Selected Medicinal Plants, Volume 4. World Health
Organization website. Available at: http://www.who.int/medicines/areas/traditional/SelectMonoVol4.pdf.
Accessed October 9, 2016.
- Dymock W, Hooper D, Warden CJH. Pharmacographia Indica. London, United
Kingdom: Kegan Paul, Trench, Trubner & Co.;1893.
- Ayurvedic
Medicinal Plants of Sri Lanka.
Available at: http://www.instituteofayurveda.org/plants/plants_list.php?s=Scientific_name.
Accessed June 2, 2017.
- Marco NM. Medicina alternativa y complementaria en la enfermedad inflamatoria
intestinal. Enfermedad
Inflamatoria Intestinal al Día.
2015. 14(2): 57-64. Available at: http://www.sciencedirect.com/science/article/pii/S1696780115000482.
Accessed August 12, 2017.
- The Plant List. Available
at: http://www.theplantlist.org/tpl1.1/record/kew-2680580.
Accessed November 8, 2016.
- International
Plant Names Index (IPNI) online database. Available at:http://www.ipni.org/ipni/idPlantNameSearch.do?id=127067-1&back_page=%2Fipni%2FeditSimplePlantNameSearch.do%3Ffind_wholeName%3Dboswellia%2Bserrata%26output_format%3Dnormal.
Accessed August 12, 2016.
- Kew
Medicinal Plant Names Services. Available at: http://mpns.kew.org/mpns-portal/searchName.
Accessed August 12, 2017.
- Paul M, Bruning G, Bergmann J, Jauch J. A thin-layer
chromatography method for the identification of three different olibanum resins
(Boswellia serrata, Boswellia papyrifera, and Boswellia carteri, respectively Boswellia sacra). Phytochem Anal. 2012;23(2):184-189.
doi: 10.1002/pca.1341.
- Catalogue of Life. Available at: http://www.catalogueoflife.org/col/details/species/id/8551582da770ae7f5d132f87ac1da7e9.
Accessed August 21, 2017.
- Indian Medicinal Plants Database:
Distribution Maps. (IMPD) Available at: http://www.medicinalplants.in/distributionmaps.
Accessed June 2, 2017.
- African Plant
Database. Available at: http://www.ville-ge.ch/musinfo/bd/cjb/africa/details.php?langue=an&id=7733.
Accessed June 12, 2017.
- African Plant
Database. Available at: http://www.ville-ge.ch/musinfo/bd/cjb/africa/details.php?langue=an&id=7737.
Accessed June 12, 2017.
- Hamm S, Bleton J, Connan J, Tchapla A. A chemical
investigation by headspace SPME and GC-MS of volatile and non-volatile terpenes
in various olibanum samples. Phytochemistry.
2005;66(12):1499-1514.
- African Plant
Database. Available at: http://www.ville-ge.ch/musinfo/bd/cjb/africa/details.php?langue=an&id=7740.
Accessed June 12, 2017.
- Niebler J, Buettner A. Frankincense revisited, Part I:
Comparative analysis of volatiles in commercially relevant Boswellia species. Chem
Biodiversity. 2016;13(5):613-629.
- Meins
J, Artaria C, Riva A, Morazzoni P, Schubert-Zsilavecz M, Abdel-Tawab M. Survey
on the quality of the top-selling European and American botanical supplements
containing boswellic acids. Planta Med.
2016; 82(6):573-579. doi:
10.1055/s-0042-103497.
- Smith
T, Kawa K, Eckl V, Morton C, Stredney R. Herbal supplements in US increase 7.7%
in 2016. HerbalGram. 2017;115:56-65.
- Goraya
GS, Ved DK. Medicinal Plants in India: An
Assessment of their Demand and Supply. New Delhi, India: National
Medicinal Plants Board, Ministry of AYUSH, Government of India, and Dehrandun,
India: Indian Council of Forestry Research & Education. 2017. Available at:
http://www.nmpb.nic.in/sites/default/files/Projects/Medicinal_Plants_in_India_An_Assessment_of_their_Demand_and_Supply.pdf.
Accessed May 22, 2018.
- Zhao Z, Chen H. Chinese Medicinal Identification. Taos,
NM: Paradigm Publications. 2014:473.
- Morrow JA. Encyclopedia of Islamic Herbal Medicine.
Jefferson, NC: McFarland & Company, Inc. 2011: 103-106.
- Adhami H-R,
Mesgarpour B, Farsam H. Herbal medicine in Iran. HerbalGram. 2007;74:34-43.
- Paul M, Bruning
G, Weihrather J. Qualitative and quantitative analysis of 17 types of tetra-
and pentacylic triterpenic acids in Boswellia
papyrifera by a semiautomatic homomodal 2D HPLC method. Chromatographia. 2001;74(1-2):29-40. doi:10.1007/s10337-011-2041-3.
- Langenheim JH. Plant
Resins: Chemistry, Evolution, Ecology, and Ethnobotany. Portland, OR:
Timber Press, 2003.
- Mertens M, Buettner A, Kirchhoff E. The volatile
constituents of frankincense: a review. Flavour
Fragr J. 2009;24(6):279-300.
- Niebler J, Buettner A.
Identification of odorants in frankincense (Boswellia sacra Flueck.) by aroma extract
dilution analysis and two-dimensional gas chromatography–mass
spectrometry/olfactometry. Phytochemistry.
2015;109:66-75.
- Woolley CL, Suhail MM, Smith BL, Boren KE, Taylor LC,
Schreuder MF, et al. Chemical differentiation of Boswellia sacra and Boswellia
carteri essential oils by gas chromatography and chiral gas
chromatography–mass spectrometry. J Chromatogr A. 2012;1261:158-163.
- Camarda L,
Dayton T, Di Stefano V, Pitonzo R, Schillaci D. Chemical composition and antimicrobial activity of some
oleogum resin essential oils from Boswellia
spp. (Burseraceae). Ann Chim. 2007;97(7):837-844.
- Chiavari G,
Galletti GC, Piccaglia R, Mohamud MA. Differentiation between resins Boswellia
carterii and Boswellia frereana (frankincense) of Somali origin. J Essent Oil Res. 1991;3(3):185-186.
- Maupetit P. New constituents in olibanum resinoid and
essential oil. Perfum Flavor.
1985;9:19-37.
- Obermann H. Die
chemischen und geruchlichen Unterschiede von Weihrauchharzen. Dragoco
Report. 1977;24:260-265.
- Baratta MT,
Dorman HJD, Deans SG, Figueiredo AC, Barroso JG, Ruberto G. Antimicrobial and antioxidant properties of some commercial essential oils.
Flavour Fragr J.
1998;13(4):235-244.
- Van Vuuren SF,
Kamatou GPP, Viljoen AM.Volatile composition and antimicrobial activity of
twenty commercial frankincense essential oil samples. S Afr J Bot. 2010;76(4):686-691.
- Kasali A, Adio
AM, Oyedeji AO, Eshilokun AO, Adefenwa M. Volatile
constituents of Boswellia serrata Roxb.
(Burseraceae) bark. Flavour
Fragr J. 2002;17(6):462-464.
- Singh B, Kumar
R, Bhandari S, Pathania S, Lai B. Volatile
constituents of natural Boswellia
serrata oleo-gum-resin
and commercial samples
. Flavour Fragr J. 2007;22(2):145-147.
- Basar S. Phytochemical investigations on
Boswellia species. PhD thesis, University of Hamburg at Hamburg, 2005.
- Chen Y, Zhou C, Ge Z, Liu Y, Liu Y, Feng W, et al. Composition
and potential anticancer activities of essential oils obtained from myrrh and
frankincense. Oncol Lett.
2013;6(4):1140-1146.
- Hayashi S,
Amemori H, Kameoka H, Hanafusa M, Furukawa K. Comparison
of volatile compounds from olibanum from various countries. J Essent Oil Res. 1998;10(1):25-30.
- Marongiu B, Piras A, Porcedda S, Tuveri E. Extraction of Santalum
album and Boswellia
carteri Birdw. volatile oil by supercritical carbon dioxide:
influence of some process parameters. Flavour Fragr J. 2006;21(4):718-724.
- Mikhaeil BR, Maatooq GT, Badria FA, Amer MM. Chemistry
and immunomodulatory activity of frankincense oil. Z Naturforsch C. 2003;58(3-4):230-238.
- Wahab SMA, Aboutabl EA, El-Zalabani SM, Fouad HA, De
Pooter HL, El-Fallaha B. The
essential oil of olibanum. Planta
Med. 1987;53(4):382-384.
- Shanmughanandhan D, Ragupathy S, Newmaster SG,
Mohanasundaram S, Sathishkumar R. Estimating herbal product authentication and
adulteration in India using a vouchered, DNA-based biological reference
material library. Drug Saf. 2016;39(12):1211-1227.
- Gafner S, Blumenthal M, Reynaud D, Foster S, Techen N. ABC
review and critique of the research article "DNA barcoding detects
contamination and substitution In North American herbal products" by
Newmaster et al. HerbalEGram.
2013;10 (11). Available at: http://cms.herbalgram.org/heg/volume10/11November/DNAbarcodingReviewandCritique.html.
Accessed June 22, 2018.
- Newmaster SG, Grguric M, Shanmughanandhan
D, Ramalingam S, Ragupathy
S. DNA barcoding detects contamination and substitution in North American
herbal products. BMC
Medicine. 2013;11:222. doi:10.1186/1741-7015-11-222.
- Mishra
P, Kumar A,
Nagireddy A, Mani DN, Shukla A, Tiwar R, et al. DNA barcoding: an efficient
tool to overcome authentication challenges in the herbal market. Plant Biotech J. 2015.doi: 10.1111/pbi.12419.
- Ayurvedic
Drugs. Available at: http://ayudrugs.blogspot.ca/2010/12/shallaki.html.
Accessed August 13, 2017.
- Somashekhar
BS, Sharma M. Training Manual for the Identification of Selected Medicinal
Plants and their Raw Drugs. Bangalore, India: Andra Pradesh State Forest
Department; 2002. Available at: http://www.forests.ap.gov.in/abkp/JFM%20CFM/CFM/Special%20Reports/FRLHT/Reports/Manual%20IDENTIFICATION.pdf.
Accessed June 18, 2018.
- United
States Pharmacopeial Convention. Boswellia
serrata. In: United States
Pharmacopoeia 40 and National Formulary 35. Rockville, MD, USA: United
States Pharmacopeial Convention, 2017.
- Council
of Europe. Indian Frankincense. In: European
Pharmacopoeia (PhEur), 8th edition. Strasbourg, France: Council
of Europe, 2014.
- Frank
A, Unger M. Analysis of frankincense from various Boswellia species with inhibitory activity on human drug
metabolizing cytochrome P450 enzymes using liquid chromatography mass
spectrometry after automated on-line extraction. J Chromatogr A. 2006;1112(1-2):255-262.
- Mathe
C, Culioli G, Archier P, Vieillescazes C. Characterization of archaeological
frankincense by gas chromatography-mass spectrometry. J Chromatogr A.
2004;1023(2): 277-285.
- Mannino G, Occhipinti A, Maffei ME.
Quantitative determination of 3-O-acetyl-11-keto-β-boswellic
acid (AKBA) and other boswellic acids in Boswellia
sacra (syn. B. carteri Birdw.)
and Boswellia serrata Roxb. Molecules. 2016;21(10):1329. doi:10.3390/molecules21101329.
REVISION SUMMARY
| Version #, Author | Date Revised | Section Revised | List of Changes |
Version 1, A. McCutcheon
| | | |