 Click here for PDF Click here for PDF
Adulteration of Milk Thistle (Silybum marianum)
By Allison McCutcheon, PhDAmerican
Botanical Council, PO Box 144345, Austin, TX 78714
Citation
(JAMA style): McCutcheon A. Adulteration of milk thistle (Silybum marianum).
Botanical Adulterants Prevention
Bulletin. Austin, TX:
ABC-AHP-NCNPR Botanical Adulterants Prevention Program; 2020. Keywords: Adulterant, adulteration, milk thistle, Silybum marianum,
silymarin, silybin, silychristin, silydianin
Goal: The goal of this bulletin is to provide timely information
on issues of adulteration of milk thistle (Silybum marianum, Asteraceae)
fruit and its extracts to the international herbal industry and extended
natural products community in general, by presenting data on the type and
occurrence of adulteration, the market situation, and consequences for the
consumer and the industry.
1
General Information
1.1 Common name: Milk thistle1
1.2 Other common names:
English:1-3 Mary’s
thistle, blessed milk thistle, spotted milk thistle,2 bull thistle,
gundagai thistle, holy thistle, lady's thistle, variegated artichoke,
variegated milk thistle,3 St. Mary's thistle
Arabic:3-4 shawk el-gamal (also written as shuk aljamal) (شوك الجمل),
shuk al-halib (شوك الحليب ), alsalabayn almurimi (السلبين
المريمي )
Armenian:5 kat’ ughtap’ush
Chinese:5 nai
ji (奶薊), shui fei ji (水飛薊)
Danish:5-6 marietidsel, mælk tidsel
Dutch:6 mariadistel
Finnish:6
maarianoh dake, hedelmä
French:3,7,8
chardon argenté, chardon Marie, lait de Notre Dame, silybe de Marie, chardon
Notre Dame, épine blanche
German:3,8
Mariendistel, Frauendistel
Greek:5
gaïdouránkatho gála (γαϊδουράγκαθο γάλα)
Hebrew:5 dilan mazui
Hindi:5 dugdh rom
Italian:6,7
cardo mariano, cardo di Maria
Japanese:5
ooazami (オオアザミ),
mariazami (マリアアザミ)
Norwegian:6
marietistel
Pharmacopoeial:8
fructus silybi Mariae, fructus cardui Mariae
Polish:6
ostropest plamisty
Portuguese:3
cardo-leiteiro
Russian:3
ostro-pestro (остро-пестро), rastoropša pyatnistaja (расторопша пятнистая)
Spanish:3,7
cardo de María, cardo lechero, cardo mariano, cardo asnal, cardo blanco,
cardo santo, poma
Swedish:3
mariatistel
1.3 Accepted Latin binomial: Silybum marianum (L.) Gaertn.
1.4 Synonyms: Carduus
marianus L., Carthamus maculatus (Scop.) Lam., Cirsium maculatum Scop., Mariana mariana (L.) Hill.,8 Silybum maculatum (Scop.) Moench., Silybum mariae (Crantz)
Grey, Silybum pygmaeum Cass.9
The synonyms S. marianum var. marianum and S. marianum var. albiflorum have been used to
differentiate respectively, the commercial, purple-flowered variety from the
white-flowered variety of milk thistle.
1.5
Botanical family: Asteraceae
1.6 Distribution
range: Indigenous
to North Africa, Asia Minor, southern Europe and southern Russian Federation;
naturalized in North and South America, Australia, China, and Central Europe.3,8
1.7 Plant part: The plant part used is the dried mature
fruit devoid of the pappus. Characteristic of the Asteraceae family, the one-ovule,
achene-like fruit is called a cypsela (pl. cypselae). However, in common
parlance (and in many scientific articles) the fruit is often referred to as a
seed. To facilitate communication and maintain continuity with the literature,
the generic terms fruit or seed are used in this document. The fruit
is comprised of four parts: pericarp, integument, albumen (storage protein),
and embryo (two large cotyledons with fat as storage material). Collectively,
the albumen and embryo are described as the kernel, while the pericarp and
integument may be called the seed coat or hull.10-12
1.8 Key constituents and markers: Silymarin,
the main pharmacologically active fraction of milk thistle,13-15 comprises 1.5–3.5% of the fruit by dry weight and 30–65% by high-performance
liquid chromatography with ultraviolet detection (HPLC-UV),16 corresponding to 65–80%
by UV/Vis of the extract.16-20 Silymarin is a complex mixture of 3-hydroxyflavonolignans (Figure 1), of which
the primary components are silybin (syn. silibin, silibinin), silychristin
(syn. silichristin), silydianin (syn. silidianin), isosilybin (syn.
isosilibin), and the flavanonol taxifolin (syn. dihydroquercetin).21-24 Silybin, isosilybin, and silychristin are each present as a pair of diastereoisomers.
The seven flavonolignans and taxifolin are the primary compounds used to assess
milk thistle quality. Silymarin is mainly found in the seed coat.25,26
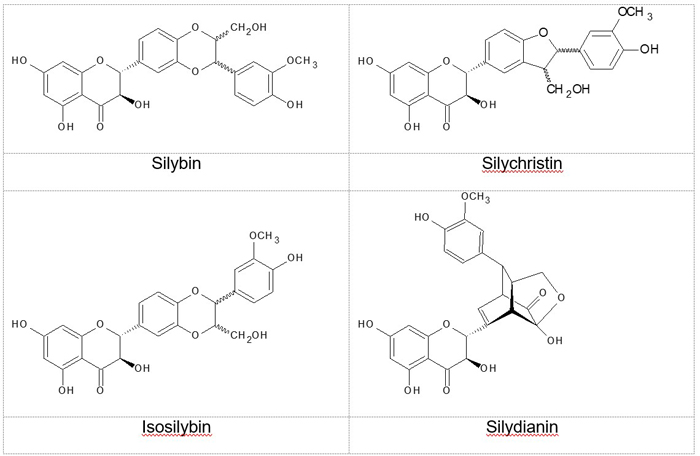
Figure 1: Principal flavonolignans in milk thistle fruit The most abundant component of silymarin, silybin (the
mixture of silybins A and B), was the first constituent that was identified,23,24 and it has been
reported as the constituent with the highest bioactivity.5,27,28 Additional
minor flavonolignans have been identified, which together with a mixture of unknown
flavonolignan oligomers and polymers constitute the remaining 20–35% of the silymarin
fraction.5,28
The foregoing data pertain to purple-flowered milk thistle. In addition to
the main silymarin constituents, a white-flowered milk thistle variety also contains the
3-deoxyflavonolignans silandrin, isosilandrin, silymonin, silyhermin, and
neosilyhermin.29-31
The
fruits also have a relatively high content of fatty oils (20–25%) that
consist of fatty acids such as linoleic acid (60–66%), oleic acid (21%),
palmitic acid (13%), phospholipids, and vitamin E.32-34
1.9 General uses and forms: The principal medicinal product is the
fruit extract standardized to contain a minimum of 45% silymarin by HPLC.16 The European Pharmacopoeia
specifies ethyl acetate, acetone, ethanol, methanol, and mixtures of water with
acetone, ethanol, and/or methanol as appropriate solvents for extraction.16 Standardized milk
thistle extract has been used for supportive treatment of acute or chronic
hepatitis and cirrhosis induced by alcohol, drugs, or toxins, treatment of dyspeptic complaints, and
gallstones.8,35 The dried extract is most commonly
sold in the form of capsules or tablets. Traditional aqueous alcohol tinctures and
extracts are also marketed but
lack scientific data to support healthy liver claims. Silybin
isolated from the silymarin extract is sold as a pharmaceutical treatment for toxic liver injury and hepatitis. The disodium
salt of silybin C-2,3-dihydrogen succinate is approved for intravenous infusion
in cases of acute death cap mushroom (Amanita phalloides, Amanitaceae)
poisoning in some European countries, and approved for conditional use (physicians
need to submit an application to
the National Institutes of Health to gain access to it) for the same indication
in the United States.36
The fruits
(whole, pulverized, and powdered) are variously sold in bulk, tea bags, capsules,
or tablets. A separate ingredient, the cold-pressed fruit oil, is sold in bulk as
an edible oil and in capsules and soft gels as a dietary supplement.37 The seed cake,
flour, and aerial plant parts may be sold as food or fodder supplements.5,37
In
addition to the medicinal uses, the photoprotective silymarin extract is also used
as cosmeceutical ingredient.37 1.10 Production and
processing: Knowledge of milk
thistle production and processing is
essential to understanding milk thistle adulteration. Milk thistle is cultivated as an annual commercial
crop. Two stable chemotypes have been described: chemotype A with a high
content of both silychristin and silybin and chemotype B with a high silydianin
content.38 A number of cultivars with a high
silymarin content and other desirable agronomic characteristics have been
developed, including Argintiu, Budakalaszi, Khoreslo, Babak Castle, Mirel, Silma,
Silyb, and Szibilla.39 In addition to genetic variables, silymarin content of the fruit can vary
significantly (0.21–17.98% of the dry weight) depending on extrinsic factors
such as geographical region, climate, growing, and harvesting conditions,40-43 and production and processing methods.5,37,44
Primary processing involves pulverization, milling, or nano-grinding, followed by
maceration or percolation of the fruit powder;5,37,44 the particle size has been shown to affect silymarin extraction efficiency.45 Silymarin is most commonly extracted from the whole pulverized fruits
or from the seed cake (the residue which remains after the oil has been cold
pressed).46 However,
silymarin may also be extracted from the other milk thistle plant parts as
follows: roots (0.05%),41 leaves (0.92%),47 flowers (0.66 %), and fruit heads
(10.67%).41 There is a developing market for these
by-products of milk thistle processing as food or fodder supplements, and for biodiesel
production.5,37 Numerous methods for extracting silymarin have been
described/patented.38,40,41,45,47-60
Most of these methods are processes involving
defatting, followed by silymarin extraction. After silymarin extraction, an additional defatting step using liquid-liquid extraction is often performed to
further reduce the oil content.61
Defatting may be
accomplished by cold pressing the oil from the fruit or solvent extraction. Cold pressing removes 60–65% of the oil. The cold pressed oil contains ~0.8–1.0 % silymarin and after further processing, it may be sold as an edible oil, dietary supplement, or
biodiesel.5,62 Another common process to remove the milk thistle seed oil is the extraction with hexane or petroleum ether, which may be followed by secondary defatting
with di-isopropyl ether. On the industrial scale, defatting using solvents is
associated with longer processing times, consumes relatively large amounts of
organic solvents, and carries the risk of excessive residual solvent presence in
the silymarin extract.63 Alternative methods that preclude the
need for defatting include hot water extraction, pressurized liquid extraction
(PLE) with either acetone, ethyl acetate, or methanol, and mechanical
separation. Hot water extraction offers a green alternative with low costs for
solvent purchase and disposal; however, the high temperatures may degrade the
silymarin.58 ,59 PLE or accelerated solvent extraction (ASE) utilizes
increased pressure to optimize extraction conditions by keeping the liquid
solvent below its boiling point at higher temperatures, and thus increasing
extraction efficiency and reducing costs for solvent purchase and disposal.56 AbouZid et al. described a mechanical
separation of the silymarin-bearing seed coat from the oil- and protein-rich kernel,
which permits the extraction of the seed coat with an appropriate solvent to
produce a silymarin extract with a high silymarin content.63
The
European Pharmacopoeia (Ph. Eur.) specifies that silymarin extract is
produced from the defatted fruit, using one or more of the following solvents:
ethyl acetate; acetone or mixture of acetone and water; ethanol or mixture of
ethanol and water; and methanol or mixture of methanol and water.16 Several studies suggest that acetone
extraction may be the most cost-effective and result in the least toxic solvent
residues.50,56,61 Isolated silymarin is an important ingredient for the herbal
drug (pharmaceutical) market and the silybin-depleted marc which still contains
some milk thistle flavonolignans reportedly is sometimes offered for sale as
silymarin extract (or simply as milk thistle seed) to the dietary supplement
industry.64-66 The sale of products with little or no flavonolignans labeled
as milk thistle seed, silymarin, or milk thistle extract is misleading and
considered fraudulent. Most all scientific literature demonstrating the
benefits of milk thistle fruit extract are based on products characterized to contain
30–65% (by HPLC) of the silymarin complex.67
By-products
of milk thistle extraction, such as the seed flour (containing ~22% protein, 7%
oil, and some residual silymarin), may be sold as a food and fodder supplement.37 However, data on therapeutic benefits
of milk thistle seed flour are lacking.
1.11 Other sources of silymarin/flavonolignans: The fruits of the related species, S. eburneum, have been reported to
contain ~10% silymarin.68 While there are limited data on the
flavonolignan composition of this species, S. eburneum can be
distinguished from milk thistle by the presence of high concentrations of isosilychristin.69 Flavonolignans that differ in their
chemical structure form silymarin compounds have been identified in members of
the plant families Asteraceae, Berberidaceae, Chenopodiaceae, Flacourtiaceae,
Fabaceae, Poaceae, and Scrophulariaceae.22,28,70,71
Silybin
has been isolated from the dried whole plant of Gentiana
apiata
(Gentianaceae) collected in Shaanxi Province, China,72 and from the fungal endophyte, Aspergillus
iizukae (Trichocomaceae), isolated from the surface-sterilized leaves of milk
thistle.73
2 Market
2.1 Importance in the trade: In 2018, milk
thistle held the eighth position among the top-forty best selling herbal
dietary supplements in the US natural channel with sales of $10,419,926, an increase of 3.5% compared to 2017.74 However, retail sales fell slightly in 2019 to the tenth
position in this channel to $10,010,699, a drop of 4.7% from 2018. Milk thistle
dietary supplements ranked 23rd in the mainstream market retail channel in 2019,
selling a measured total of $16,244,188.75
2.2
Market dynamics: Over
the past three decades, sales have remained strong, with milk thistle
consistently ranking among the forty top-selling herbal dietary supplements in the
United States, particularly in the natural sales channel. However, when comparing the annual sales
data from 2016–2019, the market has remained flat (Table 1). Both the type of milk
thistle label claims and the content declared has broadened. Silymarin content
claims range from 30–85% based on spectrophotometric ("UV/Vis") or high-performance liquid
chromatography (HPLC) assessments. There is potential confusion with respect to
levels of chemical standardization for silymarin and/or its constituents as
different analytical methods produce lower or higher readings of constituents, respectively.
Alternatively, some manufacturers specify silybin content (35–45%), some report
flavonolignan content, and others do not make any marker content claims.
Table 1: Sales data for milk thistle
dietary supplements in the United States from 2016-2019.74-77
|
Channel
|
2016
|
2017
|
2018
|
2019
|
|
|
Rank
|
Sales
[US$]
|
Rank
|
Sales
[US$]
|
Rank
|
Sales
[US$]
|
Rank
|
Sales
[US$]
|
|
Natural
|
6
|
9,968,995
|
7
|
9,960,892
|
8
|
10,419,926
|
10
|
10,010,699
|
|
Mainstream
Multi-Outlet
|
16
|
17,077,481
|
17
|
16,799,553
|
20
|
16,596,226
|
23
|
16,244,188
|
The scientific research on milk thistle over the past
three decades also has had significant impacts on market dynamics, including advances
in plant breeding and agricultural research which have led to the development
of silymarin-enriched varietals and improved agronomic techniques to increase
the silymarin content of milk
thistle fruit; processing and extraction optimization
(especially the differential extraction of only the seed coat and the resultant
increases in silymarin yield and reduction in financial and environmental
costs); and research and development of markets for milk thistle by-products.5,37 The adaptation
of these advances has allowed manufacturers to produce milk thistle extracts with
higher silymarin contents at lower cost, and access new revenue streams by
marketing milk thistle
by-products.
Costs for bulk
milk thistle extracts vary depending on the origin of the material. Authentic
European milk thistle extract is sold as an ingredient in bulk quantities for a
wholesale price of US $130-150/kg. (C. Bewicke [Ethical Naturals] email to S.
Gafner, August 11, 2020; G. Ris [Indena] email to S. Gafner, August 14, 2020) Authentic
milk thistle extracts from outside Europe complying with United States
Pharmacopeia (USP) specifications for powdered milk thistle extract20 are sold at the wholesale level for US $90-110 (Cal
Bewicke email to S. Gafner, August 11, 2020). An informal review by S. Gafner of
bulk prices for ingredients sold as containing milk thistle extracts labeled to
contain 80% silymarin on retail and e-commerce company Alibaba (Hangzhou,
China) showed costs being generally in the range of US $30-35/kg, although some
suppliers claim to offer such extracts for as little as US $1/kg.
2.3 Supply sources: Teuscher et al. stated that milk
thistle comes "from cultivated plants grown to a limited extent in
northern Germany but mainly imported from Argentina, Austria, China, Hungary, Poland,
Romania, and Venezuela."35 Vereš and Týr reported that milk
thistle is grown commercially as a medicinal plant in Europe,
Egypt, China, and Argentina.78
Some manufacturers purchase milk thistle
extracts rather than raw milk thistle fruit. As outlined in section 1.11, extraction
methods significantly impact silymarin composition and content, as well as the
cost of production. In addition to pharmacopeial 30–70% silymarin extracts of milk
thistle fruit, milk thistle by-products are also known to be sold as "milk
thistle extract." Products that do not contain the concentration and
composition of silymarin as supported by the scientific literature may not
deliver the liver-supportive benefits documented in the pharmacological and
clinical research literature and expected by consumers and/or health
professionals. Additionally, as noted, extracts prepared from by-products
claiming to provide “silymarin” and not meeting the standard definition as
established by pharmacopeial monographs and the scientific literature are considered
to be adulterated.
There have also been significant shifts
in the geographical origin of the commercial supply, and the analytical methods
used to assess silymarin content. In the 1990s and early 2000s, Europe was the
primary supplier of raw milk thistle and the analysis of silymarin content was
largely based on spectrophotometry. Since then, the market has diversified with
Asia now providing the majority of the international supply, and analytical
methods have advanced considerably. However, concerns have been expressed that
companies which market milk thistle products with label claims based only on
spectrophotometric analysis may accidentally or intentionally be selling
products that are silybin-depleted or contain <30% silymarin.65,79
3 Pharmacopeial Standards
The World Health Organization (WHO) and the USP specify the
dried mature fruit contains not less than 1.5% silymarin, calculated as
silybin.8,18 The USP
defines milk thistle extract as containing 90–110% of the claimed amount of
silymarin, calculated as silybin, consisting of the sum of silychristin and
silydianin (20–45%), silybin A and B (40–60%), and isosilybin A and B (10–20%).20 The Ph. Eur. defines the dried standardized extract as
containing "30–65%
silymarin, expressed as silibinin, and corresponding to the sum of silychristin
and silydianin (20–45% of silymarin), silybin A and B (40–60% of silymarin),
and isosilybin A and B (10–20% of silymarin)."16
4 Adulteration
4.1
Known Adulterants: Depleted
Silybum marianum extracts, Silybum
eburneum, and synthetic colorants. Globe thistle (Echinops echinatus, Asteraceae)
reportedly has been confused with milk thistle in Pakistan due to the use of
the same vernacular name “untkatara” for both milk and globe thistle in Hindi
and Urdu,80,81 but this appears to be a geographically limited issue.
4.2
Sources of information confirming adulteration
4.2.1
Chemical evidence:
According to Langhammer,82 it is difficult to discriminate between
the species S. marianum and S. eburneum based on morphological
characteristics and hence, wildcrafted material may contain the fruits of S. eburneum.
Numerous studies have found that
commercial milk thistle products do not meet their label claims for silymarin
content or pharmacopeial specifications for silymarin content.
Frommenwiler analyzed 31 milk thistle products which were acquired from the internet, local
shops, and pharmacies in the United Kingdom (UK), by high-performance
thin-layer chromatography (HPTLC).83 Among these
products, 10 had a Traditional Herbal Registration while the remaining 21
products were sold as food supplements. The 10 THR products, and seven food
supplements were deemed to be of good quality; seven additional food
supplements were consistent with the milk thistle fingerprint, except that
these products were missing the zone for taxifolin. However, four food
supplements contained a weak chromatographic fingerprint, and two did not show
any of the characteristic milk thistle zones.
Fenclova et al. used a validated
ultrahigh-performance liquid chromatography mass spectrometry (UHPLC-MS) method
to assess the quality of 26 milk thistle supplements sold as capsules* in the United
States (n = 19) and Czech markets (n = 7). Of the 24 products with silymarin
label claims, total silymarin (TS) content ranged from 35–125% of the declared
content, with four products containing <50% of the declared content and four
containing more than the declared content. The sum of flavonoid/flavonolignan content
ranged from 5–393 mg/g of capsule content, the maximum recommended daily intake
(RDI, based on product label recommendations) ranged from 18–600 mg/day, and
the sum of silybin A and B ranged from 36–66% of total silymarin (i.e., all of
the products contained silybin).84
Fibigr et al. also used a validated
UHPLC-UV method to evaluate the silymarin content of four teas and seven
supplements sold in the Czech Republic. The total silymarin content of the teas
ranged from 3.4 mg/g to 7.1 mg/g of tea. The supplements contained 39.5–77.6%
of the claimed silymarin content; the two registered pharmaceutical
preparations had the highest content (72.9% and 77.6% of the label claims). The
seven supplement samples contained 42.9–59.4% silybin, calculated as the
percentage of total silymarin.85 While some of the food supplements had much lower than
declared silymarin content, this may be partly due to different standardization
methods rather than using spent milk thistle fruit.
Pendry et al. evaluated the silymarin
content in 11 milk thistle tinctures purchased in the United UK using HPLC-UV.
The tinctures varied in their extraction ratios (4.54–18.37 mg/mL w/v) and the
percentage of alcohol (25–75%). Silymarin could not be detected in the seven samples
extracted with 25% ethanol (regardless of the extraction ratio which ranged
from 1:5 to 1:1), possibly due to the instability of flavonolignans in low
alcohol tinctures.86 Of the four samples with detectable
amounts of silymarin, only one sample with an extraction ratio of 1:1 and an
alcohol content of 70% provided an effective therapeutic dose and one sample
did not exhibit the characteristic silymarin fingerprint, suggesting the sample
was derived from an adulterant.87
Scientists at the University College of
London (UCL) evaluated seven milk thistle products sold on the European market for
a television show by the British Broadcasting Corporation (BBC) using high-performance
thin-layer chromatography (HPTLC); six of the products were legally sold in the
EU as food supplements with no silymarin label claims and one was a registered
traditional herbal medicine (THM). Two of the supplements (36%) did not contain
any milk thistle; one of these produced a faint fingerprint suggesting the presence of an
unidentified synthetic adulterant. Another two supplements contained only low
levels of milk thistle. Three products (one traditional herbal remedy and two
food supplements) produced fingerprints comparable to the standard.88 Further testing by UCL researchers
showed that of 18 commercial supplements, products sold under a traditional
herbal registration (THR) were of good quality, while 22% of food supplements
were of poor quality with little or no detectable milk thistle.89
Eight of the 12
companies ConsumerLabs tested failed testing for silymarin content in
commercial milk thistle products. Among milk thistle extract
supplements claiming to provide 80% silymarin, amounts in five products actually
ranged between 51.7–60.4% silymarin. Three other milk thistle supplements
which were tested did not list their amount of silymarin but failed to meet
minimum standards.90 Some of the discrepancies
observed are likely due to milk thistle supplement manufacturers using
spectrophotometric methods for standardization, which, as noted above, produces
a higher value for silymarin. ConsumerLab used a HPLC-UV method for the product
analysis, which produces a reading of lower numerical value, and thus,
perceptually of lesser chemical concentration.
Stranska and Hajslova used UHPLC-high
resolution mass spectroscopy (HRMS) analysis to assess the silymarin content of
milk thistle products sold in the EU. They determined that three out of seven milk
thistle powdered extract products contained ≤ 50% of the declared silymarin content.
Of the six milk thistle oil products assessed, three contained no detectable silymarin
and three contained less than 15 mg/capsule; two of the milk thistle oils
contained less than 30 mg/kg silymarin. Of the 10 milk thistle teas analyzed,
five contained <30% silymarin and only two contained ˃60% silymarin.91
Anthony and Saleh assessed 45 commercial
milk thistle products from the United States (24) and international markets (21,
purchased in Cairo, Egypt) using HPLC-MS and HPTLC. Silymarin label claims
ranged from 51.8 to 1358.0 mg/tablet. One product from the United States and
three international products did not contain any silymarin, and 23 contained <30%
silymarin (11 from the United States and 12 international). Four products from
the United States and 17 international products showed non-silymarin bands at retention
factors (Rfs) above silybin. Two US products stood out because of their
relatively high concentration of silybin, representing 78% and 93% of the total
silymarin, well above the 40–60% specified by the European and US
pharmacopeias. It is not clear how such silybin-enriched extracts are obtained,
but it could point to the addition of silybin made by chemical synthesis (a
process frequently referred to as spiking), which was published as early as
2000.92 Another explanation is the use of an
extraction process that selectively enriches silybin.93,94 Eight samples contained <100 mg/g, nine samples contained 100–200
mg/g, six contained 200–300mg/g, eight contained 300–400 mg/g, five samples
contained 400–500 mg/g, and five samples contained ˃500 mg/g.95
Lee et
al. evaluated the content of silychristin, silydianin, silybin A, silybin B, isosilybin
A, and isosilybin B in seven commercial milk thistle extracts purchased in
Philadelphia, PA using HPLC-MS. Six of the products were manufactured in the
United States and one was manufactured in China.96 Silydianin was the least abundant constituent, and the
content of this constituent did not vary significantly among the samples. There
was considerable heterogeneity among the samples in the content of silychristin,
silybin A, silybin B, isosilybin A, isosilybin B, and total silymarin. Of the
six US products, one had substantially higher levels of total silymarin, and
two products had significantly lower contents of total silymarin, while the
remaining three were intermediate and very similar in content. The Chinese extract
had the lowest contents of total silymarin. Since the authors did not divulge
the exact sample preparation procedure, only a relative comparison of silymarin
contents among the products can be made. The relative content in silybin (39–46%
of total flavonolignans) in the six US samples was relatively consistent, while
the relative silybin content in the sample from China (33.8%) was a bit lower.96
Kvasnicka
et al. used HPLC-UV to analyze the silymarin content of six commercial
silymarin extracts purchased in the EU. They reported that the total silymarin
content of the silymarin extracts ranged from 17.21–63.96%; among three
different batches of the same product, the content ranged from 41.6–63.96%. In
all of the samples, silybin made up between 40–60% of the total flavonolignans.97
Three
additional publications detailed results from quantitative analysis of
commercial milk thistle products using HPLC-UV.98-100 In two
publications, all of the analyzed
products contained significantly less silymarin than the amount claimed on the
label; however, the silybin content was between 40–60% of total flavonolignans.98,99 In the third paper, Schulz reported that the nine
commercial milk thistle products
tested contained 58–116% of the silymarin label claim, when measured by HPLC-UV.100 These findings can
be attributed in most cases to the manufacturers’ use of a spectrophotometric method
for determining silymarin content (per section 4.7 below).
A consistent theme of all these
published data on milk thistle quality is the market presence of products with
lower than declared contents in silymarin. In addition, four papers provided
evidence of milk thistle adulteration based on the absence of any of the
characteristic silymarin signals, or additional peaks/zones from undeclared
adulterants.87,88,95,101 The identity of these adulterants was not determined in
any of the four investigations.
Before the advent of official HPLC-UV
methods for the flavonolignan quantification, most manufacturers relied on
spectrophotometric methods. Some manufacturers use a direct UV-spectrophotometric method
(absorbance at 288 nm; without derivatization) for quantitative estimation of
silymarin in the extracts using silybin as the reference standard, while others
use a formerly official method which
calculates the total flavonolignan content based on the absorption of a milk
thistle extract solution after a reaction with
2,4-dinitrophenylhydrazine in alkaline conditions at 490 nm,102 or variations thereof. Many suppliers still
use spectrophotometric methods for standardization.66 Fraudsters have reportedly taken
advantage of the lack of specificity of UV/Vis tests by adding undisclosed colorants
to milk thistle extracts in order to provide an appearance of compliance with
standardization requirements.103
4.2.2
Other evidence of adulteration: There
is ample evidence (see section 4.2.1) of commercial products containing lower
amounts of silymarin. While such data do not provide direct evidence for milk
thistle adulteration, as it is possible that a manufacturer uses low-quality
starting material, leading to an extract with low amounts of flavonolignans, there
is consensus from industry experts that these low-quality products are most often
due to intentional silymarin depletion of milk thistle fruit, constituting the
fraudulent sale of a low-quality product to unsuspecting buyers.64-66,104
4.3
Accidental or intentional adulteration:
The sale of products that do not meet their silymarin label claims could be
attributed to the use of the spectrophotometric method to determine silymarin
content; however, the sale of products that do not contain any detectable
silymarin (and in some cases, contain foreign substances) cannot be accidental
unless the manufacturer(s) did not have adequate quality assurance programs. The
sale of depleted milk thistle fruit extracts is done on purpose, since such
extracts can be sold at a lower price and thus provide a competitive advantage
to fraudulent suppliers and manufacturers.64-66 Regarding
other forms of adulteration, as most of the commercial milk thistle supply is
produced under cultivation, the risk of accidental adulteration is very low;
however, the misidentification of other thistle species may occur when milk
thistle fruits are collected in the wild.80
4.4 Frequency of occurrence: The milk thistle monograph in Wichtl (2016)
stated that adulteration "practically never occurs," and in the milk
thistle fruit supply chain that may largely remain true.35 However, as summarized in section 4.2 the
evidence accumulated since 1994 suggests that 30–50% of commercial products that
are labeled to contain milk thistle extract do not meet their label
claims for silymarin content. The occurrence of products that did not contain
silymarin ranged from 0–28.6% and the incidence of products with evidence of an
adulterant ranged from 0–46%, demonstrating that the trade of substandard
extracts as an ingredient is extensive.
4.5
Possible safety/therapeutic issues: While
the sale of milk thistle products with sub-therapeutic levels of silymarin pose
no immediate safety concerns, the therapeutic impacts are substantial, considering
that consumers who use milk thistle may have serious liver disease or liver
dysfunction and may not obtain the benefits expected. Additionally, the presence
of unidentified adulterants may potentially pose safety issues. As Spink and Moyer point out, the act
of adulteration, although carried out with economic or financial motivation, can have an
effect that can lead unintentionally to a public health concern.105
4.6
Analytical methods to detect adulteration: For whole fruit, botanical identity may be confirmed by a
qualified analyst based on organoleptic and macroanatomical characteristics.
The identity of powdered material may be determined using a combination of
organoleptic, microscopic, and chemical techniques. Both the Ph. Eur. and USP specify HPTLC methods for the identification
of milk thistle fruits,18,19 powdered milk
thistle fruit,106 and powdered
extracts.16,20 Langhammer
reported a simple chemical test with sulfuric acid to differentiate S.
marianum and S. eburneum.82 but the sensitivity to identify mixed batches may
be lacking. There are numerous
chemical techniques that can be used to authenticate milk thistle extracts,
including TLC,107 HPTLC,20 HPLC-UV,44,85,86,108 HPLC-MS,84,86,96,109,110 mid- and near-infrared spectroscopy (MIR and NIR),111,112 nuclear magnetic resonance (NMR),108 and various combinations of these methods. Quantitation of silymarin in a given extract is
complicated by the fact that part of what is considered to be silymarin is a
mixture of flavonolignans. Though
they differ slightly, the Ph. Eur. and USP provide official HPLC-UV methods for
authentication of milk thistle extracts and quantification of silymarin content.
Silymarin depletion and silybin spiking can be determined by measuring the
individual flavonolignan concentrations, e.g., using HPLC-UV. However, other forms of
adulteration such as the presence of dyes may not be detected unless the
methods have been specifically developed to do so.113,114 Genetic approaches, such as random
amplified polymorphic DNA (RAPD) analysis,115-117 have focused mainly on the distinction among milk thistle
genotypes, but such methods could be used as well to differentiate milk thistle
and closely related species. Since the same DNA is found in all parts of a
specific plant species, DNA-based identification methods cannot distinguish
between genuine and depleted milk thistle extracts.
4.7
Perspectives: A number of hypotheses have been put
forth to explain the frequent occurrence of milk thistle products containing <30% silymarin, including
silybin depletion, the use of spectrophotometry to quantify silymarin content, milk
thistle by-products, dyes, and excipients. Silybin depletion is a reasonable explanation, because
silybin is
easily isolated from silymarin extract by precipitation, isolated silybin has a
much higher market value than silymarin, and the silybin-depleted marc might be
sold inappropriately as silymarin extract. However, none of the studies
described in section 4.2.1 reported evidence supporting this hypothesis (i.e.,
all of the products that contained silymarin also contained silybin in
appropriate proportions to the other marker constituents). Another explanation
is the sale of silymarin as a whole to the pharmaceutical industry, while the
leftover, post-extract milk thistle fruit mass (marc) might be re-extracted and
sold to the dietary supplement and cosmetic industries.
When the label in commercial products claims
80% silymarin, it is indicative that the number is based on analysis by UV/Vis
spectrophotometry and not HPLC-UV. The high incidence of milk thistle products
containing <30% silymarin reported in the published literature has also been
attributed to the use of spectrophotometry to analyze silymarin content. The 2,4-dinitrophenylhydrazine method assesses the content of all
ketones/flavones in milk
thistle samples, not only the flavonolignans that constitute
the silymarin complex. Hence, this technique typically overestimates silymarin
content.65,79,118
Direct analysis at 288 nm usually provides even higher “silymarin” contents as
many compounds absorb at this wavelength and thus interfere with the assay. In
addition, spectrophotometric
analysis may not detect other forms of adulteration, such as the presence of milk
thistle by-products, other herbs with high flavone/flavonolignan content, or
dyes. For these reasons, HPLC-UV analysis superseded spectrophotometry for the
authentication of milk thistle extracts. The
substitution/addition of non-functional plant parts and the deliberate
mislabeling of these products is one of the oldest forms of adulteration.113 The entry of milk
thistle
by-products into the commercial supply chain, together with the increase in the
complexity of supply networks, has created an environment where these
inexpensive, "near-identical" milk thistle materials that
may pass both spectrophotometric and cursory chromatographic analyses can effectively
be sold as " milk thistle extracts."119 The presence of milk
thistle
by-products in commercial milk thistle preparations may also account for
the appearance of HPTLC bands that do not occur with authentic fruit extracts. The increasing incidence of unauthorized
colorants/dyes in herbs and spices has been ranked as a serious hazard.120 In the absence of analytical methods
specifically designed for their detection, this form of adulteration may go
undetected,101,114,121-123 especially when combined with milk thistle by-products.
A largely unexplored
explanation of finding such a large array of substandard products is the
presence of large amounts of excipients in finished milk thistle products. Silymarin has poor oral
bioavailability and a number of novel formulations specifically designed to
enhance bioavailability have been developed.124-135 However, the impact of
these excipients/processes on the efficiency of silymarin extraction in the
gastrointestinal tract has not been investigated. Considering the issue from a geographical
perspective, China has become a leading supplier of milk thistle and several authors have reported that "Chinese"
milk thistle extracts contain <30% silymarin. However, this
constitutes weak evidence when the low number of samples that have been
analyzed (<10) are considered in relation to the high volume of milk thistle
that China produces. More rigorous research with representative sampling is needed
before this postulate can be given credence.
Unfortunately, most of the
investigators reporting the occurrence of milk
thistle products
containing <30% silymarin did not conduct in-depth analyses of the
actual chemical composition of the suspect products. In the absence of further
evidence, it is unclear which of these hypotheses may be correct; however,
considering the current domination of HPLC analysis in the industry, it strains
credibility to accept that the recently reported deficiencies may be blamed on
spectrophotometric overestimation of silymarin content.
Further, none of these postulates can explain the alarmingly high
incidence of milk thistle products that do
not contain any silymarin.
5 Conclusions
Regardless of the underlying cause, the relatively
high incidence of materials labeled as milk
thistle
that contain little or no silymarin is clearly an ongoing problem, a problem
that is legally defined within some countries as food fraud.104,136,137 Lack of adequate therapeutic
benefit in patients believing that they can utilize milk thistle for serious
liver disease, and loss of consumer confidence due to products that do not meet
their label claims are serious concerns. The sale of products labeled as
containing milk thistle but lacking the
claimed ingredient and containing unidentified adulterants instead potentially may also
pose a health concern. However, it is a risk which may be prevented with
appropriate supply chain qualification and adequate quality control, and quality
assurance protocols.
* The RDIs for milk thistle issued by the
European Medicines Agency6 varies depending on the extraction
solvent and the drug-extract ratio. However, the lowest RDI is generally above
200 mg. For comparison, the RDI for the most extensively clinically tested milk
thistle product, Legalon® (Meda Pharma AB, Solna, Sweden) is standardized to
420 mg silymarin per day.
6 References
McGuffin M, Kartesz JT, Leung AY,
Tucker AO. American Herbal Products Association's Herbs of Commerce. 2nd ed. Silver Spring, MD: American Herbal
Products Association; 2000. Silybum
marianum (L.) Gaertn. Integrated Taxonomic Information System (ITIS) online
database; 2020. https://www.itis.gov/servlet/SingleRpt/SingleRpt?search_topic=TSN&search_value=38413#null. Accessed June 5, 2020.
Silybum
marianum (L.) Gaertn. USDA, Agricultural Research Service, National Plant
Germplasm System. Germplasm Resources Information Network (GRIN-Taxonomy);
2020. https://npgsweb.ars-grin.gov/gringlobal/taxonomydetail.aspx?id=33952. Accessed June 5, 2020.
سلبين مريمي. Wikimedia Foundation, Inc.
https://ar.wikipedia.org/wiki/%D8%B3%D9%84%D8%A8%D9%8A%D9%86_%D9%85%D8%B1%D9%8A%D9%85%D9%8A. Accessed July 16, 2020.
Chambers CS, Holečková V, Petrásková L,
et al. The silymarin composition… and why does it matter??? Food Res Int. 2017;100:339-353.
European Union herbal monograph on Silybum marianum (L.) Gaertn., fructus.
In: London UK, ed. European Medicines Agency Committee on Herbal Medicinal
Products (HMPC): European Medicines Agency; 2018.
Invasive Species Compendium. Silybum marianum. Centre for Agriculture
and Bioscience International (CABI). https://www.cabi.org/isc/datasheet/50304. Accessed June 5, 2020.
Fructus Silybi mariae. WHO Monographs on Selected Medicinal Plants.
Vol 2. Geneva, Switzerland: World Health Organization; 2002:300-316.
Silybum
marianum (L.) Gaertn. The Plant List. Version 1.1; 2013. http://www.theplantlist.org/tpl1.1/record/gcc-114114. Accessed June 5, 2020.
Cappelletti EM, Caniato R. Silymarin
localization in the fruit and seed of Silybum
marianum L. Gaertn. Herba Hungarica. 1984;23:53-66.
Stoiljković Z, Petrović S, Ilić B.
Examination of localization of silymarin and fatty oil in Silybum marianum (L.) Gaertn. fruit. Chem Ind Chem Eng Q 2007;13:55-59.
Kaczmarek F, Mrugasiewicz K, Lutomski J,
Gorecki P, Scigacz M, Kotlarek B, Inventors. Sposob wytwarzania z nasion
ostropestu plamistego (Silybum marianum
(L.) Gaertn.) koncentratu o duż ej zawartos´ci sylimaryny. [Method of
manufacturing a concentrate with a high content of silymarin from milk thistle
(Silybum marianum (L.) Gaertn.)
seeds.]. 1978.
Wagner H, Hörhammer L, Seitz M. Chemical
evaluation of a silymarin-containing flavonoid concentrate from Silybum marianum (L.) Gaertn. [German]. Arzneimittel-Forsch. 1968;18(6):696-698.
Wagner H, Hörhammer L, Münster R. On the
chemistry of silymarin (silybin), the active principle of the fruits from Silybum marianum (L.) Gaertn. (syn. Carduus marianus L.) [German]. Arzneimittel-Forsch. 1968;18(6):688-696.
Wagner H, Diesel P, Seitz M. Zur Chemie
und Analytik von Silymarin aus Silybum
marianum Gaerth [German]. Arzneimittel-Forsch.
1974;24:466-470.
Silybi mariani extractum siccum
raffinatum et normatum. European
Pharmacopoeia (Ph. Eur. 10.0). Strasbourg, France: European Directorate for
the Quality of Medicines and Health Care; 2020:1501-1502.
Morazzoni P, Bombardelli E. Silybum marianum (Carduus marianus). Fitoterapia.
1995;66:3-42.
United States Pharmacopeia. Milk thistle.
USP43-NF38. Rockville, MD: United
States Pharmacopeial Convention; 2020.
Silybi mariani fructus. European Pharmacopoeia (Ph. Eur. 10.0).
Strasbourg, France: European Directorate for the Quality of Medicines and
Health Care; 2020:1499-1501.
United States Pharmacopeia. Powdered milk
thistle extract. USP 43-NF 38.
Rockville, MD: United States Pharmacopeial Convention; 2020.
Kim NC, Graf TN, Sparacino CM, Wani MC,
Wall ME. Complete isolation and characterization of silybins and isosilybins
from milk thistle (Silybum marianum).
Organic & biomolecular chemistry. 2003;1(10):1684-1689.
Kaloga M. Isosilychristin, ein neues
Flayonolignan aus Silybum marianum L. Gaertn. / Isosilychristin, a New
Flavonolignan from Silybum marianum L.Gaertn [German]. Z Naturforsch B. 1981;36(2).
Pelter A, Hänsel R. Struktur des
Silybins: I. Abbauversuche [German]. Chemische
Berichte. 1975;108(3):790-802.
Pelter A, Hänsel R. The structure of
silybin (silybum substance E6), the first flavonolignan. Tetrahedron Lett. 1968;9(25):2911-2916.
Giuliani C, Tani C, Maleci Bini L, Fico
G, Colombo R, Martinelli T. Localization of phenolic compounds in the fruits of
Silybum marianum characterized by
different silymarin chemotype and altered colour. Fitoterapia. 2018;130:210-218.
Hlangothia D, Abdel-Rahman FH, NguyÅn To,
Anthony K, Saleh MA. Distribution of silymarin in the fruit of Silybum marianum L. Pharm Anal Acta. 2016;7:1-4.
Biedermann D, Vavříková E, Cvak L, Křen
V. Chemistry of silybin. Nat Prod Rep. 2014;31(9):1138-1157.
Csupor D, Csorba A, Hohmann J. Recent
advances in the analysis of flavonolignans of Silybum marianum. J Pharm
Biomed Anal. 2016;130:301-317.
Szilági I, Tétényi P, Antus S, et al.
Struktur von Silandrin und Silymonin, zwei neuen Flavanolignanen aus einer
weißblühenden Silybum marianum
Varietät [German]. Planta Med. 1981;43(2):121-127.
Samu Z, Nyiredy S, Baitz-Gács E, et al.
Structure elucidation and antioxidant activity of (-)-isosilandrin isolated
from Silybum marianum L. Chem Biodiversity. 2004;1(11):1668-1677.
Nyiredy S, Samu Z, Szücs Z, Gulácsi K,
Kurtán T, Antus S. New insight into the biosynthesis of flavanolignans in the
white-flowered variant of Silybum
marianum. J Chromatogr Sci. 2008;46(2):93-96.
Harrabi S, Romdhane H, Daassa M, Fellah
H. Fatty acid and triacylglycerol compositions of milk thistle seeds growing
wild in Tunisia (Silybum marianum
L.). Acta Aliment Hung. 2015;44:304-310.
Hadolin M, Škerget M, Knez Ze, Bauman D.
High pressure extraction of vitamin E-rich oil from Silybum marianum. Food Chem.
2001;74(3):355-364.
Yin QF, Wang SH, Nan JX, Li SY. The fatty
acid compositions of Silybum marianum by
GC–MS. J Yanbian Univ (Nat Sci). 1998;24:26-28.
Teuscher E, Willuhn G, Loew D. Silybi
mariani fructus. In: Blaschek W, ed. Wichtl
- Teedrogen und Phytopharmaka. Stuttgart, Germany: Wissenschaftliche
Verlagsgesellschaft mbH; 2016:612-615.
Goetz G. Milk thistle extract combats
mushroom poisoning. Food Safety News
[online] 2011.
Andrzejewska J, Martinelli T, Sadowska K.
Silybum marianum: non-medical exploitation of the species. Ann Appl Biol. 2015;167(3):285-297.
Martinelli T, Whittaker A, Benedettelli
S, Carboni A, Andrzejewska J. The study of flavonolignan association patterns
in fruits of diverging Silybum marianum
(L.) Gaertn. chemotypes provides new insights into the silymarin biosynthetic
pathway. Phytochemistry. 2017;144:9-18.
Ali A, Anestis K, Reza S. Breeding
objectives and selection criteria for milk thistle [Silybum marianum (L.) Gaertn.] improvement. Notulae Botanicae Horti Agrobo. 2013;41(2).
Poppe L, Petersen M. Variation in the
flavonolignan composition of fruits from different Silybum marianum chemotypes
and suspension cultures derived therefrom. Phytochemistry.
2016;131:68-75.
Martin RJ, Lauren DR, Smith WA, Jensen
DJ, Deo B, Douglas JA. Factors influencing silymarin content and composition in
variegated thistle (Silybum marianum).
NZ J Crop Hort Sci. 2006;34:239-245.
Karkanis A, Bilalis D, Efthimiadou A.
Cultivation of milk thistle (Silybum
marianum L. Gaertn.), a medicinal weed. Ind
Crops Prod. 2011;34(1):825-830.
Radjabian T, Huseini HF.
Anti-hyperlipidemic and anti-atherosclerotic activities of silymarins from
cultivated and wild plants of Silybum
marianum L. with different content of flavonolignans. Iran J Pharm Therapeut. 2010;9(2):63-67.
AbouZid SF, Chen S-N, Pauli GF. Silymarin
content in Silybum marianum populations
growing in Egypt. Ind Crops Prod. 2016;83:729-737.
Saleh IA, Kamal SA, Shams KA, Abdel-Azim
NS, Aboutabl EA, Hammouda FM. Effect of particle size on total extraction yield
and silymarin content of Silybum marianum
L. seeds. Res J Pharm Biol Chem Sci. 2015;6(2):803-809.
El Sherif F, Khattab S, Ibrahim AK, Ahmed
SA. Improved silymarin content in elicited multiple shoot cultures of Silybum marianum L. Physiology and molecular biology of plants : an international journal
of functional plant biology. 2013;19(1):127-136.
Omar AA, Hadad GM, Badr JM. First
detailed quantification of silymarin components in the leaves of Silybum
marianum cultivated in Egypt during different growth stages. Acta Chromatograph. 2012;24:463-474.
Saleh IA, Vinatoru M, Mason TJ,
Abdel-Azim NS, Aboutabl EA, Hammouda FM. Ultrasonic-assisted extraction and
conventional extraction of silymarin from Silybum
marianum seeds; A comparison. Res J
Pharm Biol Chem Sci. 2015;6(2):709-717.
Elwekeel A, El-Fishawy AM, AbouZid SF.
Silymarin content in Silybum marianum
fruits at different maturity stages. J
Med Plant Res. 2013;7:1665-1669.
Leko V, Inventor; Leko, V., assignee.
Method for isolation of silymarin from Silybum
marianum seeds. 2008.
Carrier DJ, Crowe T, Sokhansanj S, Wahab
J, Barl B. Milk thistle, Silybum marianum
(L.) Gaertn., flower head development and associated marker compound profile. J Herbs Spices Med Plants. 2003;10(1):65-74.
Subramaniam S, Vaughn K, Carrier DJ,
Clausen EC. Pretreatment of milk thistle seed to increase the silymarin yield:
an alternative to petroleum ether defatting. Bioresource technology. 2008;99(7):2501-2506.
Gupta GK RS, Rao, PR. Isolation of
antihepatotoxic agents from seeds of Silybum
marianum. Res Ind. 1982;27:37-42.
Andrzejewska J, Sadowska K, Mielcarek S.
Effect of sowing date and rate on the yield and flavonolignan content of the
fruits of milk thistle (Silybum marianum
L. Gaertn.) grown on light soil in a moderate climate. Ind Crops Prod. 2011;33(2):462-468.
Kahol AP, Singh KL, Tandon S, Kumar S,
Inventors; Council of Scientific and Industrial Research (CSIR), assignee.
Process for isolation of hepatoprotective agent silymarin from the seeds of the
plant Silybum marianum. 2001.
Wianowska D, Wiśniewski M. Simplified
procedure of silymarin extraction from Silybum
marianum L. Gaertner. J Chromatogr
Sci. 2015;53(2):366-372.
Wallace S, Carrier DJ, Beitle RR, Clausen
EC, Griffis CL. HPLC-UV and LC-MS-MS characterization of silymarin in milk
thistle seeds and corresponding products. J
Nutraceut Funct Med Foods. 2003;4(2):37-48.
Duan L, Carrier DJ, Clausen EC. Silymarin
Extraction from Milk Thistle Using Hot Water. Paper presented at: Proceedings
of the Twenty-Fifth Symposium on Biotechnology for Fuels and Chemicals.2004;
Breckenridge, CO.
Barreto JF, Wallace SN, Carrier DJ,
Clausen EC. Extraction of nutraceuticals from milk thistle: I. Hot water
extraction. Applied Biochemistry and Biotechnology.
2003;105 -108:881-889.
Keshavarz Afshar R, Chaichi MR, Ansari
Jovini M, Jahanzad E, Hashemi M. Accumulation of silymarin in milk thistle
seeds under drought stress. Planta. 2015;242(3):539-543.
Intelmann D, Karlseder A, Inventors;
Bionorica SE, assignee. Milk thistle extract of fruit shells of Silybum marianum, process of manufacture
and use. 2015.
Szczucinska A, Lipkowski AW, Baranowska
B, Walisiewicz-Niedbalska W, Rożycki K, Maciuszczak-Kotlarek H. Utylizacja
odpadu nasion ostropestu plamistego. I. Olej z ostropestu plamistego jako
antyutleniacz / Utilisation of milk thistle seed waste. Part I. Milk thistle
oil as antioxidant [Polish]. Ro´sliny
Oleiste - Oilseed Crops. 2003;25:717-724.
AbouZid SF, Chen S-N, McAlpine JB,
Friesen JB, Pauli GF. Silybum marianum
pericarp yields enhanced silymarin products. Fitoterapia. 2016;112:136-143.
Depletion. Adulteration of Natural Extracts and Quality Challenges. East
Windsor, NJ: Sabinsa; 2018:12.
Gorman R. Adulteration...an ingredient
checklist. Nutraceuticals now [online].
Inverness, Scotland: Johnson-Johnson Publishing; 2016.
Anonymous. Not all milk thistle extracts
are made equal: Analytical methods, USP standards, and adulteration. TherapeuticFocus [online]. 2018;5(1).
Assessment report on Silybum marianum (L.) Gaertn., fructus. London, United Kingdom:
European Medicines Agency Committee on Herbal Medicinal Products (HMPC);
2016:1-79.
Halbach G, Winkler W. Notizen: Flavonoide
Inhaltsstoffe in den Früchten von Silybum
eburneum / Flavonoids in the Fruits of the Genus Silybum eburneum [German]. Z
Naturforsch B. 1971;26(9):971-972.
Adzet T, Iglesias J, Martinez F.
Flavonolignans in the fruits of Silybum
genus taxa: a chromatographic and mass spectrometric survey. Plant Med Phytother. 1993;26(2):117-129.
Vue B, Chen QH. The potential of
flavonolignans in prostate cancer management. Curr Med Chem. 2016;23(34):3925-3950.
Begum SA, Sahai M, Ray AB.
Non-conventional lignans: Coumarinolignans, flavonolignans, and
stilbenolignans. In: Kinghorn AD, Falk H, Kobayashi J, eds. Fortschritte der Chemie organischer
Naturstoffe / Progress in the Chemistry of Organic Natural Products, Vol. 93.
Vienna, Austria: Springer Vienna; 2010:1-70.
Zhou L, Li XK, Miao F, et al. Further
studies on the chemical constituents of Chinese folk medicine Gentiana apiata N.E. Br. Journal of Asian Natural Products Research. 2009;11(4):345-351.
El-Elimat T, Raja HA, Graf TN, Faeth SH,
Cech NB, Oberlies NH. Flavonolignans from Aspergillus
iizukae, a fungal endophyte of milk thistle (Silybum marianum). J Nat
Prod. 2014;77(2):193-199.
Smith T, Gillespie M, Eckl V, Knepper J,
Reynolds CM. Herbal supplement sales in US increase by 9.4% in 2018. HerbalGram. 2019;123:62-73.
Smith T, May G, Eckl V, Reynolds CM. US sales
of herbal supplements increase by 8.6% in 2019. HerbalGram. 2020;127:54-67.
Smith T, Kawa K, Eckl V, Morton C,
Stredney R. Herbal supplement sales in US increase 8.5% in 2017, topping $8
billion. HerbalGram. 2018;119:62-71.
Smith T, Kawa K, Eckl V. Herbal
supplement sales in US increase 7.7% in 2016. HerbalGram. 2017;115:56-65.
Vereš T, Týr Š. Milk thistle (Silybum marianum (L.) Gaertn.) as a weed
in sustainable crop rotation. Res J Agric
Sci. 2012;44:118-122.
Smoller N. Milk thistle: Quality update.
Vol 2020. Woodstock, NY: Woodstock Vitamins Blog (formerly Village Vitality);
2015.
Ahmad M, Khan MA, Hasan A, Zafar M,
Sultana S. Chemotaxonomic standardization of herbal drugs milk thistle and
globe thistle. Asian J Chem. 2008;6(20):4443-4459.
Plant Details for a Echinops echinatus ROXB. Institute of Trans-Disciplinary Health
Sciences & Technology (TDU) and Foundation for Revitalisation of Local
Health Traditions (FRLHT); 2020. http://envis.frlht.org/plantdetails/6abcf64dea9e5749f127dcd581bd4944/23f1315ef624d31a7138e3fce39a90b4. Accessed August 24, 2020.
Langhammer L. Anatomie und Histochemie
der Früchte von Silybum. Planta Med. 1969;17(3):268-275.
Frommenwiler DA, Sharaf MHM, Reich E. The
truth behind herbal products: how HPTLC can help herbal industry detect
adulteration? Planta Med. 2019;85(18):ISL
EA-06.
Fenclova M, Novakova A, Viktorova J, et
al. Poor chemical and microbiological quality of the commercial milk
thistle-based dietary supplements may account for their reported unsatisfactory
and non-reproducible clinical outcomes. Sci
Rep. 2019;9(1):11118.
Fibigr J, Šatínský D, Solich P. A new
approach to the rapid separation of isomeric compounds in a Silybum marianum extract using UHPLC
core-shell column with F5 stationary phase. J
Pharm Biomed Anal. 2017;134:203-213.
Bilia AR, Bergonzi MC, Gallori S, Mazzi
G, Vincieri FF. Stability of the constituents of calendula, milk-thistle and
passionflower tinctures by LC-DAD and LC-MS. J Pharm Biomed Anal. 2002;30(3):613-624.
Pendry BA, Kemp V, Hughes MJ, et al.
Silymarin content in Silybum marianum
extracts as a biomarker for the quality of commercial tinctures. J Herbal Med. 2017;10:31-36.
Trust me – I’m a doctor. Do herbal
supplements contain what they say on the label? . British Broadcasting
Corporation (BBC) Two; 2015. http://www.bbc.co.uk/programmes/articles/4hX30rMYkMv9YjMTH38MY6/do-herbal-supplements-contain-what-they-say-on-the-label. Accessed September 23, 2019.
Booker A, Heinrich M. Value chains of
botanicals and herbal medicinal products: A European perspective. HerbalGram. 2016;112(40-45).
Milk thistle and liver formula supplements
review. ConsumerLab.com; 2019. https://www.consumerlab.com/reviews/milk_thistle_and_liver_supplements/milkthistle/. Accessed June 16, 2020.
Stranska M, Hajslova J. Herbal based
dietary supplements and other food products: Assessment of quality and chemical
safety. Institutional Cooperation of UCT Prague + UiT Tromsø; 2016; Tromsø,
Norway.
Gu W, Chen X, Pan X, Chan ASC, Yang T-K.
First enantioselective syntheses of (2R,3R)- and
(2S,3S)-3-(4-hydroxy-3-methoxyphenyl)-2-hydroxymethyl-1,4-benzodioxan-6-carbaldehyde.
Tetrahedron: Asymmetry. 2000;11(13):2801-2807.
Tan C, Xu X, Shang Y, Fu X, Xia G, Yang
H. A novel approach for the efficient extraction of silybin from milk thistle
fruits. Pharmacogn Mag. 2014;10(40):536-540.
De Iasi G, Feola M, Di Manzano CM,
Inventors; Istituto Biochimico Italiano Giovanni Lorenzini S.P.A., assignee. An
oral pharmaceutical formulation comprising silybin. 2017.
Anthony K, Saleh MA. Chemical profiling
and antioxidant activity of commercial milk thistle food supplements. J Chem Pharm Res. 2012;4(10):4440-4450.
Lee JI, Narayan M, Barrett JS. Analysis
and comparison of active constituents in commercial standardized silymarin
extracts by liquid chromatography-electrospray ionization mass spectrometry. J Chromatogr B. 2007;845(1):95-103.
Kvasnička F, Bíba B, Ševčík R, Voldřich
M, Krátká J. Analysis of the active components of silymarin. J Chromatogr A. 2003;990(1-2):239-245.
Eklund L, Simon JP, Ballenger J. High
performance liquid chromatography of flavonolignans in commercial milk thistle
supplements. BIOS. 2009;80(4):164-169.
Liu H, Du Z, Yuan Q. A novel rapid method
for simultaneous determination of eight active compounds in silymarin using a
reversed-phase UPLC-UV detector. J
Chromatogr B. 2009;877(32):4159-4163.
Schulz H-U, Schürer M, Krumbiegel G,
Wächter W, Weyhenmeyer R, Seidel G. Untersuchungen zum Freisetzungsverhalten
und zur Bioäquivalenz von Silymarin-Präparaten. Arzneimittel-Forsch. 1995;45:61-64.
Frommenwiler DA, Reich E, Sudberg S, Sharaf
MHM, Bzhelyansky A, Lucas B. St. John's wort versus counterfeit St. John's
wort: An HPTLC study. J AOAC Int. 2016;99(5):1204-1212.
Monographie über Mariendinstelfrüchte. Deutsches Arzneibuch 10 (DAB 10), Band 3.
Stuttgart, Germany & Frankfurt, Germany: Deutscher Apotheker Verlag &
Govi Verlag; 1993.
Gafner S. Botanical ingredient adulteration
– how some suppliers attempt to fool commonly used analytical techniques. 30th
International Horticultural Congress; 2018; Istanbul, Turkey.
Chapter 9 - Federal Food, Drug, and
Cosmetic Act, Subchapter IV - Food, Section 342 - Adulterated food. United States Code, 2006 Edition, Supplement
5, Title 21 - Food and Drugs. Washington, DC: U.S. Government Publishing
Office; 2006.
Spink J, Moyer DC. Understanding and
combating food fraud. Food Tech. 2013;67(1):30-35.
United States Pharmacopeia. Powdered milk
thistle. USP43-NF38. Rockville, MD:
United States Pharmacopeial Convention; 2020.
Wagner H, Bladt S. Plant Drug Analysis: A Thin Layer Chromatography Atlas. Berlin,
Germany: Springer 2009.
Cheilari A, Sturm S, Intelmann D, Seger C,
Stuppner H. Head-to-head comparison of ultra-high-performance liquid
chromatography with diode array detection versus quantitative nuclear magnetic
resonance for the quantitative analysis of the silymarin complex in Silybum marianum Fruit Extracts. J Agric Food Chem. 2016;64(7):1618-1626.
Graf TN, Cech NB, Polyak SJ, Oberlies NH. A
validated UHPLC-tandem mass spectrometry method for quantitative analysis of
flavonolignans in milk thistle (Silybum marianum) extracts. J Pharm Biomed Anal. 2016;126:26-33.
Shibano M, Lin A-S, Itokawa H, Lee K-H.
Separation and characterization of active flavonolignans of Silybum marianum by liquid
chromatography connected with hybrid ion-trap and time-of-flight mass
apectrometry (LC–MS/IT-TOF). J Nat Prod. 2007;70(9):1424-1428.
Zavoi S, Fetea F, Ranga F, Pop RM, Baciu A,
Socaciu C. Comparative fingerprint and extraction yield of medicinal herb
phenolics with hepatoprotective potential, as determined by UV-Vis and FT-MIR
spectroscopy. Notulae Botanicae Horti
Agrobotanici Cluj-Napoca. 2011;39(2).
Vágnerová L, Bradáčová M, Pluháčková H. The
determination of contained compounds in milk thistle [Silybum marianum L. (Gaertn.)] by the means of FT-NIR. MendelNet. 2016;23:168-172.
Galvin-King P, Haughey SA, Elliott CT. Herb
and spice fraud; the drivers, challenges and detection. Food Control. 2018;88:85-97.
Bessaire T, Savoy MC, Mujahid C, Tarres A,
Mottier P. A new high-throughput screening method to determine multiple dyes in
herbs and spices. Food Addit Contam Part
A Chem Anal Control Expo Risk Assess. 2019;36(6):836-850.
Ražná K, Hlavačková L, Bežo M, et al.
Application of the RAPD and miRNA markers in the genotyping of Silybum marianum (L.) Gaertn. Acta Fytotechnica et Zootechnica (online). 2015;18(4):83-89.
AbouZid S. Authentication of Silybum marianum varieties using RAPD
analysis. Plant Tissue Cult Biotechnol. 2014;24(1):57-63.
Hamouda M. Molecular analysis of genetic
diversity in population of Silybum
marianum (L.) Gaertn in Egypt. J
Genet Eng Biotechnol. 2019;17(1):12.
AbouZid S. Silymarin, natural
flavonolignans from milk thistle In: Rao V, ed. Phytochemicals: A Global Perspective of Their Role in Nutrition and
Health. Rijeka, Croatia: InTech; 2012:255-272.
Eliot C. The new phenomenon of criminal
fraud in the food supple chain. NSF Int
Rep. 2014:1-187.
van Asselt ED, Banach JL, van der
Fels-Klerx HJ. Prioritization of chemical hazards in spices and herbs for
European monitoring programs. Food
Control. 2018;83:7-17.
Dixit S, Khanna SK, Das M. A simple
2-directional high-performance thin-layer chromatographic method for the
simultaneous determination of curcumin, metanil yellow, and Sudan dyes in
turmeric, chili, and curry powders. J
AOAC Int. 2008;91(6):1387-1396.
Petrakis EA, Cagliani LR, Tarantilis PA,
Polissiou MG, Consonni R. Sudan dyes in adulterated saffron (Crocus sativus L.): Identification and
quantification by 1H NMR. Food
Chem. 2017;217:418-424.
Oplatowska-Stachowiak M, Elliott CT. Food
colors: Existing and emerging food safety concerns. Crit Rev Food Sci Nutr. 2017;57(3):524-548.
Voinovich D, Perissutti B, Magarotto L,
Ceschia D, Guiotto P, Bilia AR. Solid state mechanochemical simultaneous
activation of the constituents of the Silybum
marianum phytocomplex with crosslinked polymers. J Pharm Sci. 2009;98(1):215-228.
Voinovich D, Perissutti B, Grassi M,
Passerini N, Bigotto A. Solid state mechanochemical activation of Silybum marianum dry extract with
betacyclodextrins: Characterization and bioavailability of the coground
systems. J Pharm Sci. 2009;98(11):4119-4129.
Liang J, Liu Y, Liu J, et al.
Chitosan-functionalized lipid-polymer hybrid nanoparticles for oral delivery of
silymarin and enhanced lipid-lowering effect in NAFLD. J Nanobiotechnol. 2018;16(1):64.
Javed S, Kohli K, Ali M. Reassessing
bioavailability of silymarin. Alt Med
Rev. 2011;16(3):239-249.
Tung N-T, Tran C-S, Nguyen H-A, et al.
Formulation and biopharmaceutical evaluation of supersaturatable
self-nanoemulsifying drug delivery systems containing silymarin. Int J Pharmaceut. 2019;555:63-76.
Nasr SS, Nasra MMA, Hazzah HA, Abdallah OY.
Mesoporous silica nanoparticles, a safe option for silymarin delivery:
preparation, characterization, and in vivo evaluation. Drug Deliv Transl Res. 2019;9(5):968-979.
Méndez-Sánchez N, Dibildox-Martinez M,
Sosa-Noguera J, Sánchez-Medal R, Flores-Murrieta FJ. Superior silybin
bioavailability of silybin–phosphatidylcholine complex in oily-medium soft-gel
capsules versus conventional silymarin tablets in healthy volunteers. BMC Pharmacol Toxicol. 2019;20(1):5.
Yousaf AM, Malik UR, Shahzad Y, Mahmood T,
Hussain T. Silymarin-laden PVP-PEG polymeric composite for enhanced aqueous
solubility and dissolution rate: Preparation and in vitro characterization. J Pharm Anal. 2019;9(1):34-39.
Ibrahim AH, Rosqvist E, Smått J-H, et al.
Formulation and optimization of lyophilized nanosuspension tablets to improve
the physicochemical properties and provide immediate release of silymarin. Int J Pharmaceut. 2019;563:217-227.
Theodosiou E, Purchartová K, Stamatis H,
Kolisis F, Křen V. Bioavailability of silymarin flavonolignans: Drug
formulations and biotransformation. Phytochem
Rev. 2014;13(1):1-18.
Kosina P, Kren V, Gebhardt R, Grambal F,
Ulrichová J, Walterová D. Antioxidant properties of silybin glycosides. Phytother Res. 2002;16 Suppl 1:S33-39.
Barzaghi N, Crema F, Gatti G, Pifferi G,
Perucca E. Pharmacokinetic studies on IdB 1016, a silybin- phosphatidylcholine
complex, in healthy human subjects. European
journal of drug metabolism and pharmacokinetics. 1990;15(4):333-338.
Food Chemical Codex. Appendix XVII: Food
fraud mitigation guidance. FCC 10.
Rockville, MD: United States Pharmacopeial Convention; 2016.
Morin
J-F, Lees M. Definition of food fraud and food authenticity. In: Morin J-F,
Lees M, eds. FoodIntegrity Handbook.
La Chapelle sur Erdre, France: Eurofins Analytics France; 2018:XIII-XVII.
REVISION SUMMARY
|
Version #, Author,
|
Date Revised
|
Section Revised
|
List of Changes
|
|
Version 1, Allison McCutcheon
|
n/a
|
n/a
|
n/a
|
|