AUGUST 2015
Bilberry Fruit Extract Laboratory Guidance Document
By Stefan Gafner, PhD
Chief Science Officer, American Botanical Council Technical Director, ABC-AHP-NCNPR Botanical Adulterants ProgramKeywords: Adulterant, adulteration, anthocyanin, bilberry, synthetic dye, Vaccinium myrtillus, Vaccinium spp.
1. Purpose
2.
Scope
3.
Common and Scientific Names
3.1
Common Name
3.2
Other Common Names
3.3 Latin Binomial
3.4 Synonyms
3.5
Botanical Family
4.
Botanical Description
Table
1.
Known bilberry adulterants of plant origin:
Scientific names, family, and common names
5.
Identification and Distinction Using Macroanatomical Characteristics
6.
Identification and Distinction Using Microanatomical Characteristics
7.
Genetic Identification and Distinction
8.
Chemical Identification and Distinction
8.1
Chemistry of Vaccinium myrtillus and
the Potential Adulterants
Figure
1: Anthocyanins occurring in bilberry fruits
Table
2: Phenolic acid, anthocyanin, and flavonol marker compounds in berries and
fruit other than bilberry and related Vaccinium
spp.*
8.2
Laboratory Methods
8.2.1
HPTLC
Figure 2: HPTLC
analysis of bilberry fruit extract, bilberry fruit, cranberry fruit, blueberry
fruit, and acerola cherry (Malpighia
sp., Malpighiaceae) fruit according to reference 74; Detection: visible light. Lane 2: cyanidin-3-O-glucoside
chloride; lane 3: cyanidin chloride.
Image provided by CAMAG (Muttenz,
Switzerland)
Figure 3: HPTLC
analysis of bilberry fruit extract, bilberry fruit, cranberry fruit, blueberry
fruit, and acerola cherry fruit using the stationary and mobile phase specified
in reference 74; Detection: anisaldehyde reagent, viewed under UV light at 366
nm. Lane 2: cyanidin-3-O-glucoside
chloride; lane 3: cyanidin chloride. Image provided by CAMAG (Muttenz,
Switzerland)
Figure
4: HPTLC analysis of bilberry fruit extract, bilberry fruit, cranberry fruit,
blueberry fruit, and acerola cherry fruit using the stationary and mobile phase
specified in reference 74; Detection: anisaldehyde reagent. Lane
2: cyanidin-3-O-glucoside chloride;
lane 3: cyanidin chloride. Image provided by CAMAG (Muttenz, Switzerland)
8.2.2
HPLC and UHPLC
Figure
5. HPLC-UV chromatogram of an authentic bilberry extract analyzed according to
the conditions outlined in the European
Pharmacopoeia; Image provided by Indena S.p.A. (Milan, Italy)
8.2.3
UV/Vis Spectrophotometry
Table
3. Comparison among the different approaches to authenticate bilberry
9.
Conclusion
10. References
Appendix
1
Table
4: Comments on the published HPLC methods to authenticate bilberry extracts and
detect adulteration
Table
5. Comparison of different published HPLC methods for V. myrtillus. Sample preparation steps and times are indicated only
for dry bilberry extracts, not for fresh fruit or fruit juice
1.
Purpose
Market demand for bilberry (Vaccinium
myrtillus, Ericaceae) fruit extracts,
combined with high prices and falling profit margins have resulted in
unscrupulous manufacturers selling various ingredients labeled “bilberry
extract.” Adulteration predominantly occurs with anthocyanin-rich extracts from
other species, e.g., bog bilberry (V. uliginosum),
lingonberry (V. vitis-idaea), European
elder (Sambucus nigra, Adoxaceae),
and Chinese mulberry (Morus australis, Moraceae). Additional adulterants
reportedly include black soybean (Glycine
max, Fabaceae) hull or black rice
(Oryza sativa, Poaceae) extracts, and
synthetic colorants like amaranth dye, an azo dye prohibited for use by the United
States Food and Drug Administration (FDA) as a suspected carcinogen, and/or
charcoal.1 This Laboratory Guidance Document presents a review of
the various analytical technologies and methods used to differentiate between
authentic bilberry extracts and potential adulterants.
2.
Scope
Previous
pharmacopeial test methods for bilberry fruit extract based on UV/Vis absorption of the extract (spectrophotometric methods) are acceptable for
quantification of total anthocyanidins, but have proven insufficient to detect
adulteration with anthocyanin-rich extracts from other species or synthetic
dyes; therefore, other analytical techniques must be used to comply with the
legal requirement (for example, according to the Good Manufacturing Practice
rule in the United States, and in other countries) to confirm the identity of bilberry
fruit extracts. This review is a compilation of published analytical methods for
bilberry fruit extracts, and an evaluation of the utility of each method to
authenticate bilberry extracts or to detect potential adulterants. This Laboratory
Guidance Document does not cover the
analysis of bilberry leaves or bilberry leaf
extracts but may have
applications for other anthocyanin-rich berry ingredients, some of which are also
known to have quality issues. Analysts can use this review to help guide the
appropriate choice of techniques and methods for their specific bilberry materials
intended for resale or use in consumer products. A positive assessment of a
specific method for testing V. myrtillus
fruit extracts in their particular matrix
in this Laboratory Guidance Document does not remove the responsibility of quality
control and laboratory personnel to demonstrate adequate method performance in
their own laboratory (and/or in a qualified third-party contract laboratory) using
accepted protocols outlined in the Good Manufacturing Practices for dietary
supplements in the United States (21 CFR Part 111) and/or by AOAC International,
International Organization for Standardization (ISO), the World Health
Organization (WHO), and the International Conference on Harmonisation (ICH).
3.
Common and Scientific Names
3.1
Common Name: Bilberry2
3.2
Other Common Names
English:
European blueberry, whortleberry, huckleberry
French:
Myrtille, gueule-noire, raisin des bois, vigne des montagnes, ambroche,
ambreselle, brimbelle
German:
Heidelbeere, Blaubeere, Schwarzbeere, Waldbeere, Bickbeere, Moosbeere
Italian:
Mirtillo, ampulette, asaire, bagole, baggiole, cesarelle, giasine,
lambrune, murucule
Spanish: Arándano
azul, mirtilo
Chinese: Hei
guo yue ju (黑果越桔)
3.3 Latin Binomial: Vaccinium
myrtillus L.
3.4 Synonyms: Vaccinium myrtillus var. oreophilum (Rydb.) Dorn; Vaccinium myrtillus subsp. oreophilum (Rydb.) Á. Löve, D. Löve & B.M. Kapoor; Vaccinium oreophilum Rydb.; Vaccinium
myrtillus var. microphyllum Hook.; Vaccinium yatabei
Makino3,4
3.5
Botanical Family: Ericaceae
4.
Botanical Description
Botanical descriptions for V. myrtillus and its adulterant species
are provided in local, national, and international floras and selected
publications, e.g., by Ritchie.5 Identifying and differentiating
between V. myrtillus and related species
requires personnel trained in botany for the assessment of materials with
intact botanically characteristic features.
Table 1.
Known bilberry adulterants of plant origin:
Scientific names, family, and common names
|
Speciesa
|
Synonym(s)a
|
Family
|
Standardized common nameb
|
Other common namesc-e
|
|
Aronia
melanocarpa (Michx.)
Elliott
|
Aronia arbutifolia var. nigra (Willd.) F.Seym.;
A. nigra (Willd.) Britton; Mespilus arbutifolia var. nigra (Willd.) Britton; Photinia
melanocarpa (Michx.) K.R.Robertson & J.B.Phipps;
Pyrus arbutifolia var. nigra Willd.;
Pyrus melanocarpa (Michx.) Willd.;
Sorbus melanocarpa (Michx.) Heynh.
|
Rosaceae
|
Not
established
|
Black
chokeberry
|
|
Glycine max (L.) Merr.
|
Dolichos soja L.;
Glycine angustifolia Miq.; G. gracilis Skvortsov;
G. hispida (Moench) Maxim.;
Phaseolus max L.;
Soja japonica Savi;
S. soja H.Karst.;
S. viridis Savi
|
Fabaceae
(Leguminosae)
|
Soy bean
|
Sojabean,
soya
bean,
da
dou (大豆)
|
|
Morus australis Poir.
|
Morus acidosa Griff.;
M. bombycis Koidz.;
M. cavaleriei H. Lév.;
M. formosensis Hotta;
M. hastifolia F.T. Wang & T. Tang ex Z.Y. Cao;
M. inusitata H. Lév.;
M. longistylus Diels;
M. nigriformis (Bureau) Koidz.
|
Moraceae
|
Not
established
|
Chinese
mulberry,
ji
sang (鸡桑)
|
|
Morus nigra L.
|
|
Moraceae
|
Not
established
|
Black mulberry,
purple mulberry,
hei
sang (黑桑)
|
|
Oryza sativa L.
|
Oryza communissima Lour.;
O. formosana Masam.& Suzuki;
O. glutinosa Lour.;
O. montana Lour.;
O. plena (Prain) N.P.Chowdhury;
O. praecox Lour.;
O. rubribarbis (Desv.) Steud.
For a complete list, see references 3
and 6.
|
Poaceae
|
Rice
|
Upland rice,
dao (稻)
|
|
Prunus avium (L.) L.
|
Cerasus avium (L.) Moench;
Druparia avium (L.) Clairv.
|
Rosaceae
|
Sweet
cherry
|
Bird
cherry, mazzard cherry,
wild
cherry
|
|
Ribes nigrum L.
|
Botrycarpum nigrum (L.) Spach;
B. nigrum (L.) A. Rich.; Grossularia nigra (L.) Rupr.;
Ribes cyathiforme Pojark.;
R. olidum Moench;
R. pauciflorum Turcz. ex Ledeb.;
Ribesium nigrum (L.) Medik.
|
Grossulariaceae
|
Black
currant
|
Cassis,
European black currant, garden black currant,
quinsy
berries, squinancy berries,
hei
cha biao zi (黑茶藨子)
|
|
Rubus idaeus L.
|
Rubus
acanthocladus Borbs;
R. buschii (Rozanova)
Grossh.;
R. chrysoscarpus Čelak. ex Gyer;
R. × euroasiaticus Sinkova;
R. fragrans Salisb.;
R. frambaesianus Lam.;
R. obtusifolius Willd.;
R. sericeus Gilib.
For a complete list, see references 3
and 6.
|
Rosaceae
|
Raspberry
|
Red raspberry, fu pen zi
(复盆子)
|
|
Sambucus nigra L.
|
Sambucus graveolens Willd.
|
Adoxaceae
|
European
elder
|
Black elder, black-berried
alder,
boor tree,
bountry, ellanwood, ellhorn
|
|
Vaccinium angustifolium Aiton
|
Cyanococcus angustifolius (Aiton)
Rydb.
|
Ericaceae
|
Blueberryf
|
Lowbush
blueberry
|
|
Vaccinium corymbosum L.
|
Cyanococcus corymbosus (L.) Rydb.
|
Ericaceae
|
Blueberryf
|
Highbush
blueberry, giant whortleberry
|
|
Vaccinium oxycoccos L.
|
Oxycoccus oxycoccos (L.)
MacMill.;
O. palustris Pers.;
O. quadripetalus Schinz
& Thell.;
O.
quadripetalus Gilib.; O. vulgaris Hill;
Schollera oxycoccos (L.)
Roth.
For a complete list, see references 3
and 6.
|
Ericaceae
|
Cranberry
|
Small
cranberry, hong mei tai zi (红莓苔子)
|
|
Vaccinium uliginosum
L.
|
Myrtillus uliginosus (L.) Drejer;
Vaccinium gaultherioides Bigelow;
V. occidentale A. Gray;
V. pedris Holub;
V. pubescens Wormsk. ex Hornem.
|
Ericaceae
|
Not
established
|
Bog
blueberry, bog bilberry, northern bilberry,
du si yue ju
(笃斯越桔)
|
|
Vaccinium vitis-idaea L.
|
Rhodococcum vitis-idaea Avrorin;
Vaccinium
jesoense Miq.;
Vitis-idaea punctata Moench
|
Ericaceae
|
Lingonberry
|
Alpine
cranberry, cowberry, foxberry, lingberry, lingenberry, northern mountain
cranberry,
red bilberry, whortleberry, yue
ju (越桔)
|
aThe
Plant List and the Tropicos database.3,6 A comprehensive list of synonyms can be accessed through both websites.
bThe American Herbal Products Association’s
Herbs of Commerce, 2nd ed.
(2000).2
cHerbs of Commerce, 2nd ed.,2 the USDA GRIN Database,4 the USDA PLANTS Database,7
and the Health Canada website.8
dFlora of China.9
ePDR for Herbal Medicines
, 2nd ed.10
fThere are differences in the meaning of “blueberry” and “wild blueberry.” In the US dietary supplement trade, the
name “blueberry” is restricted to three species, Vaccinium angustifolium, V.
corymbosum, and V. pallidum.2
In Europe, V. myrtillus is often called blueberry, though bilberry is
the English word which refers to this species in the trade.2 The
hybrid cultivated blueberries from which the majority of the commercial food
supply is derived are generally called blueberries. According to Steven Foster, president of Steven Foster Group, Inc., North American wild blueberry,
common blueberry, common lowbush blueberry, low sweet blueberry, and lowbush blueberry refer to V. angustifolium which is common in the Northeastern
United States and is commercially harvested in its habitat. Velvet leaf blueberry (V. myrtilloides) is also traded as “wild blueberry,” and is mostly wild-harvested in the
Canadian maritime provinces. It is safe to assume that “wild blueberry” in a
commercial sense refers to both V. angustifolium and V. myrtilloides
(e-mail communication, July 1, 2015).
In
addition to the species listed in Table 1, synthetic dyes, charcoal, and
anthocyanin-rich extracts from other berries (in particular, extracts
manufactured in China made from unidentified berries) were also reported as
adulterants of bilberry extracts.11
Sections 5-8 of this Laboratory
Guidance Document discuss macroscopic, microscopic, genetic, and chemical
authentication methods for V. myrtillus.
A comparison among the various approaches is presented in Table 3 at the end of
section 8.
5.
Identification and Distinction Using Macroanatomical Characteristics
Since bilberries are not
cultivated,11 all commercially available bilberry fruits are
wildcrafted; this means that companies are unable to grow their own crop and
must rely on a thorough identity testing program to ensure that the correct wild-harvested
material is purchased. Macroscopic identification criteria of bilberry fruits
can be helpful for companies that purchase the dried fruits to make an extract.
Descriptions on macroscopic identification have been published, e.g., in the American
Herbal Pharmacopoeia (AHP) monograph by Upton,12 in the European
Pharmacopoeia,13 and in the book Herbal
Drugs and Phytopharmaceuticals.14 The AHP monograph contains a table
with criteria to distinguish V. myrtillus
from V. uliginosum and V. vitis-idaea. A comparison between V. myrtillus and Aronia
melanocarpa (Rosaceae) is given by Filippini et al.15
However, a comprehensive macroscopic description of other closely related Vaccinium species (e.g., the North
American blueberry species) is lacking. Macroscopic test methods are obviously
inadequate to detect adulteration of bilberry extracts. For correct
authentication, additional means of testing (e.g., chemical) should be used.
6.
Identification and Distinction Using Microanatomical Characteristics
Detailed
microscopic descriptions of V. myrtillus
are found in several references.12,13,16,17 The AHP monograph also contains
microscopic data on two known adulterants, bog bilberry (V. uliginosum) and lingonberry (V.
vitis-idaea).12 A paper by Villani et al. compares the
microanatomical characteristics of bilberry fruit and European elder fruit,18
but based on the available authoritative resources, there is no reference to find information on V.
myrtillus and other (i.e., in addition to European elder) known
adulterants, e.g., black soybean hull or Morus
australis.
Comments:
While microscopic distinction of a blueberry powder could be helpful to detect
adulteration with synthetic dyes, charcoal, or powders from a different berry
source, the use in authentication or detection of adulteration of bilberry
extracts is limited. (It is conceivable that a synthetic dye or charcoal could
be detected as an adulterant of a bilberry extract by microscopy, but no papers
in this regard have been located at the time of the publication of this
document.) Furthermore, criteria for the identification of a number of
adulterant species are lacking. In addition, typical microanatomical features
are absent in extracts of bilberry fruit. Therefore, the use of microscopy for
the authentication of bilberry extracts and for the detection of its
adulterants is generally considered inadequate.
7.
Genetic Identification and Distinction
One method described in the
literature was evaluated in this review: Jaakola et al.19
Comments: High-resolution
melting (HRM) of amplicons is a rapid DNA barcoding method that works with
samples consisting of fresh and dry material from a single species with intact
DNA. The method was able to distinguish bilberries from lingonberry, bog
bilberry, blueberry (V. corymbosum × V. angustifolium), crowberry (Empetrum nigrum, Ericaceae), gooseberry
(Ribes uva-crispa, Grossulariaceae),
honeysuckle (Lonicera caerulea,
Caprifoliaceae), and mountain shadbush (Amelanchier
bartramiana, Rosaceae). However, the method is not applicable to bilberry
extracts since DNA is often damaged (denatured or fragmented) and/or removed
via filtration during the extraction process, even though newer DNA methods
have shown some success with dried extracts. Also, a genetic assessment is not
able to determine the plant part, which is a legal requirement of dietary
supplement ingredient identification. Therefore, DNA-based methods are of
limited use for bilberry extract authentication or detection of the presence of
adulterants.
8.
Chemical Identification and Distinction
A number of analytical
methods have been published for identifying V.
myrtillus berry extracts, including compendial methods, e.g., by the European
Pharmacopoeia13 or the United
States Pharmacopeia.20 These methods are cited in the Laboratory
Methods section below. Distinction based on the phytochemical profile requires
knowledge of the composition of bilberry fruit extracts and its adulterants.
The important components in V. myrtillus and
its adulterating species are listed below with an emphasis on anthocyanins.
Obviously, the composition of extracts can vary greatly depending on the
manufacturing process.
8.1
Chemistry of Vaccinium myrtillus and
the Potential Adulterants
Vaccinium myrtillus: Dry bilberry fruit contains
up to 10% of catechin-type tannins (min. 1% according to PhEur),13
proanthocyanidins, and anthocyanins. The anthocyanins are mainly glucosides,
galactosides, or arabinosides of delphinidin, cyanidin, and – to a lesser
extent – malvidin, peonidin, and petunidin (Figure 1).21 However,
there are considerable differences in the quantitative composition of
anthocyanins, e.g., glucosides are almost completely absent in some samples
from Eastern Finland.22 Flavonols include quercetin- and kaempferol-glycosides.
The fruits also contain other phenolic compounds (e.g., chlorogenic acid,
caffeic acid, o-, m-, and p-coumaric acids, and ferulic acid), citric and malic acids, and
volatile compounds.12,14 Marker compounds that can be used to detect
adulteration with other berry or fruit extracts are indicated in Table 2.
 Figure
1: Anthocyanins occurring in bilberry fruits
Figure
1: Anthocyanins occurring in bilberry fruits
Aronia melanocarpa: Black
chokeberry fruit contains up to 5.2% proanthocyanidins and up to 2% anthocyanins,23
mainly cyanidin-3-O-galactoside, with
lesser amounts of cyanidin-3-O-arabinoside,
-xyloside, and -glucoside. [24,25] Other flavonoids include glycosides of
quercetin.26 The fruit also contains other phenolic compounds (chlorogenic
acid and neochlorogenic acid), malic and citric acids, and volatile compounds.23
The much simpler and very different anthocyanin pattern of chokeberry fruit can
be used to distinguish it from bilberry fruit. In addition, the presence of
high amounts of neochlorogenic acid or quercetin-3-O-rhamnosyl-(1→6)-galactoside is indicative of adulteration with Aronia spp. or berries from other
species.
Glycine max: Dry
hydroalcoholic extracts of soybean contain mainly sugars (58-65%), proteins
(5-7%), lipids (4-7%), minerals (7-10%) and saponins (6-10%). The content of
the characteristic isoflavones in crude extracts is between 0.8 and 2%,27
with 6''-O-malonylgenistin,
6''-O-malonyldaidzin, genistin, and
daidzin as the quantitatively most important.28,29 A study comparing
soybean with variously colored seed coats determined that only black seed coat
soybeans contain cyanidin-3-O-glucoside.
[30] A specific extract of the black soybean hull was shown to contain 39.7%
proanthocyanidins, 9.2% cyanidin-3-O-glucoside,
and 6.2% catechin.31 Besides cyanidin-3-O-glucoside, which makes up to 80% of anthocyanins in black soybean,
delphinidin-3-O-glucoside (ca. 13%) and
petunidin-3-O-glucoside (3-4%) were quantitatively
next, while six other anthocyanins were found in very low amounts.28
Analysis of a black soybean hull market sample confirmed cyanidin-3-O-glucoside as the major anthocyanin and
peonidin-3-O-glucoside as a minor
component.32 The presence of
isoflavones and the different anthocyanin pattern, dominated by cyanidin-3-O-glucoside but lacking the anthocyanin-arabinosides,33
allow a distinction between black soybean hull and
bilberry extracts.
Morus australis: The
fruits of Morus australis are rich in anthocyanins, predominantly cyanidin-3-O-rutinoside,
but also cyanidin-3-O-glucoside, pelargonidin-3-O-glucoside, the alkaloid
1-deoxynojirimycin, and the flavonoid rutin.34,35 The anthocyanidin-rutinosides and 1-deoxynojirimycin are absent in Vaccinium berries
and can be used as markers to detect adulteration. Rutinosides of cyanidin and pelargonidin
are reportedly good markers for adulteration since they occur in many Morus species
(e.g., M. atropurpurea, M.
alba, and M.
nigra).21
Morus nigra: Black
mulberry is a good source of organic acids and phenolics. The fruit contains
3.5-19.9% malic acid and 0.6-2.3% citric acid.36 Chlorogenic acid (0.05-0.14%)
was found to be the prominent phenolic acid, while rutin (0.07-0.21%) is the
major flavonoid. The main anthocyanins are cyanidin-3-O-glucoside (0.01-0.70%) and cyanidin-3-O-rutinoside (0.005-0.57%). The concentrations of pelargonidin-3-O-glucoside and pelargonidin-3-O-rutinoside are below 0.03% for both
fresh and dry mulberry.37-39 In addition, the berry contains the
alkaloid 1-deoxynojirimycin, which was found in juices of eight different
mulberry species (at concentrations between 30 and 80 mg/mL), including M. nigra.35 This alkaloid, or
cyanidin-3-O-rutinoside and
pelargonidin-3-O-rutinoside, can be
used as markers for adulteration with this and other mulberry species.
Oryza sativa: The composition of various rice
parts (endosperm, bran, and hull) has been the subject of a number of reviews.40,41
Rice hulls consist mainly of lignin, hemicellulose, cellulose, and hydrated
silica.42 Cyanidin-3-O-glucoside
and peonidin-3-O-glucoside are the
main anthocyanins of black rice extract.32 Anthocyanin content in
rice bran strongly depends on color, with black rice bran having the most,
followed by purple, red, and brown rice bran. The major anthocyanin of rice
bran is cyanidin-3-O-glucoside,
accounting for 51-84% of the total, followed by peonidin-3-O-glucoside (6-16%), cyanidin-3-O-rutinoside
(3-5%), and cyanidin-3-O-galactoside
(1-2%).41 The major flavones in bran are tricin, luteolin, and
apigenin, with tricin found in unpigmented rice hulls. No flavones have been
reported from pigmented hulls. Other compounds in bran and hulls are phenolic
acids (e.g., ferulic acid, p-coumaric
acid, sinapic acid) and tocopherols.41 An indication of bilberry
extract adulteration with pigmented rice could be the presence of a large amount
of cyanidin-3-O-glucoside, although
other extracts (e.g., made from soybean hulls or European elder berries) would
lead to a similar outcome. Tricin (5,7,3'-trihydroxy-2',4'-dimethoxyflavone),
cyanidin-3-O-rutinoside, or large
amounts of cyanidin-3-O-glucoside can
be used to detect adulteration with rice bran extracts.
Prunus avium:
Sweet cherry fruit contains high levels of sugars and sugar alcohols, with up
to 8.9 g, 7.6 g, and 6.8 g/100 g fresh fruit for glucose, fructose, and
sorbitol, respectively.43 Other important constituents are the
polyphenols, especially anthocyanins, phenolic acid derivatives (predominantly
neochlorogenic acid, with lower amounts of chlorogenic acid and caffeoylquinic
acid), catechin, epicatechin, and rutin.43-45 The main anthocyanin
is cyanidin-3-O-rutinoside with 5.7-128.9
mg/100 g fresh weight (fw), followed by cyanidin-3-O-glucoside (0.4-34.8 mg/100 g fw) and peonidin-3-O-rutinoside (0.01-8.4 mg/100 g fw). Other
anthocyanins reported from wild cherries are peonidin-3-O-glucoside and peonidin-3-O-rutinoside.43-45
The presence of cyanidin-3-O-rutinoside
and pelargonidin-3-O-rutinoside,
while not exclusive for sweet cherry, is an indication of bilberry extract
adulteration with other materials.
Ribes nigrum:
Black currant fruit contains high levels of polyphenols, especially
anthocyanins, phenolic acid derivatives (both hydroxybenzoic and
hydroxycinnamic acids), flavonols (glycosides of myricetin, quercetin,
kaempferol, and isorhamnetin), and proanthocyanidins (between 120 and 166
mg/100 g fresh berries).46-47 The main anthocyanins are
delphinidin-3-O-rutinoside and
cyanidin-3-O-rutinoside, but
delphinidin- and cyanidin-3-O-glucoside
are also found.47-49 The best markers for the presence of extracts
made from berries of R. nigrum are delphinidin-, cyanidin-, and
myricetin-3-O-rutinoside.21
Rubus
spp.:
Due to the large number of distinct species and hybrids, it is beyond the scope
of this Laboratory Guidance Document to provide a comprehensive phytochemical
review of all Rubus spp. The
conclusions regarding the composition of Rubus
spp. in this paragraph are based on review articles by Lee et al.50
and Kaume et al.51 Fresh blackberry (Rubus spp. according to Kaume et al.)51 fruit contains
over 88% water, 5.3% total fiber, 4.9% total sugar (mainly glucose and
fructose), 1.4% protein, and 0.5% total lipids. Total
anthocyanins reportedly vary between 38-326 mg/100 g fw in blackberry samples.51
Phenolic acids (free and conjugated forms of hydroxycinnamic and hydroxybenzoic
acids), catechin, epicatechin, and flavonol-glycosides (quercetin- and
kaempferol-glycosides) make up the phenolic monomers that have been reported in
Rubus fruits. Typically, these
compounds are less abundant than the phenolic polymers (ellagitannins) or
anthocyanins.50 Anthocyanins from Rubus fruits are mainly derivatives of cyanidin with non-acylated
glycosyl moieties; however, anthocyanins containing acylated sugars such as
cyanidin-3-O-malonylglucoside and
cyanidin-3-O-dioxalylglucoside can be
found occasionally, e.g. in blackberries, at low concentrations. Cyanidin-3-O-glucoside and cyanidin-3-O-rutinoside appear to be common to all Rubus spp., but vary with regard to the
relative amounts. Cyanidin-3-O-rutinoside,
also occurring in mulberry and cherry species (Table 2), is not found in
bilberry fruit, and can be used as a marker compound for adulteration. In black
raspberries, cyanidin-3-O-xylosylrutinoside
is the predominant anthocyanin.50 The presence of this anthocyanin
is indicative for either black raspberry or red currant (Ribes rubrum, Grossulariaceae) fruit (Table 2).
Sambucus nigra: European elder berries are also rich in polyphenolic
compounds. The anthocyanin content is dominated by cyanidin-3-O-glucoside and cyanidin-3-O-sambubioside, with lesser amounts of
cyanidin-3-O-sambubioside-5-O-glucoside and cyanidin-3,5-O-diglucoside.24,47,52 Other
phenolic compounds occurring in European elder berries are chlorogenic acid,
rutin, and smaller amounts of
isoquercitrin.52 The proanthocyanidin content was established in one
publication as 23 mg/100 g fresh black elder berries.47 Black elder
berries can be distinguished from bilberry by the presence of cyanidin-3-O-sambubioside and cyanidin-3-O-sambubioside-5-O-glucoside.
Vaccinium angustifolium:
The qualitative composition of lowbush blueberries is quite similar to that of
bilberry. According to Primetta,21 the content of chlorogenic acid
is higher in lowbush and highbush blueberries when compared to bilberry. The
total anthocyanin content is lower in blueberries than in bilberries. However,
blueberry (highbush and lowbush) has a higher relative malvidin content.21
Kalt reported the presence of anthocyanins with acetylated sugar moieties, with
malvidin-3-O-acetylgalactoside and
malvidin-3-O-acetylglucoside being
most abundant, in lowbush and velvet leaf (V.
myrtilloides) blueberries.53 The occurrence of eight different
anthocyanin-acetylglycosides in lowbush blueberry was reported by Wu and Prior.54
Therefore, these acetylated anthocyanins can be used as marker compounds to
detect the presence of lowbush blueberry extracts.
Vaccinium corymbosum:
Highbush blueberries also have a chemical composition that is very similar to
bilberry. The variability in the anthocyanin pattern among highbush blueberries
cultivated in various geographic locations and those collected in the wild, as
outlined by Kalt,53 makes a distinction based on chemical markers
particularly difficult. Highbush blueberries reportedly contain higher amounts
of chlorogenic acid,21 but this compound alone is not a suitable
marker for adulteration. The presence of acetylated anthocyanins, which can be
used as markers for adulteration with lowbush and velvet leaf blueberry
species, has been reported by several authors,53,55 but appears to
be inconsistent.53 However, the relative amount of malvidin-3-O-glucoside and malvidin-3-O-galactoside – which are among the
major anthocyanins in blueberries but are less abundant in bilberry fruit,
where delphinidin- and cyanidin-glycosides are predominant – can be used as a criterion
to indicate substitution or admixture of blueberry (highbush and lowbush) material.11,53,56
Vaccinium oxycoccos:
Compared to bilberry fruit, cranberry contains relative high amounts of
flavonols, mainly galactosides and other glycosides of quercetin and myricetin,
but low amounts of phenolic acids (caffeic acid, ferulic acid, and p-hydroxybenzoic acid).57,58
The contents of organic acids in freeze-dried cranberry from Poland was 14.7%,
7.5%, and 5.8% for citric, malic, and quinic acids, respectively,59
and has been found to be higher in cranberry juice compared to bilberry and
blueberry juices.60 Cranberry from Western Canada contained five
major anthocyanins, with cyanidin-3-O-glucoside,
cyanidin-3-O-arabinoside, cyanidin-3-O-galactoside, peonidin-3-O-galactoside, and peonidin-3-O-arabinoside in order of decreasing quantities.61
The most abundant anthocyanins in cranberries from Finland were cyanidin-3-O-arabinoside (23.1% of total
anthocyanins), peonidin-3-O-galactoside
(21.5%), cyanidin-3-O-galactoside
(19.2%), and peonidin-3-O-arabinoside
(14.1%).62 Based on the available data on anthocyanins, cranberry
extracts can be distinguished from bilberry extracts by the absence of
delphinidin- and pelargonidin-glycosides and the presence of relatively high
amounts of peonidin-3-O-galactoside
and peonidin-3-O-arabinoside.
Vaccinium uliginosum: The
qualitative composition of bog blueberries is quite similar to that of
bilberry, but there are some quantitative differences that can be used to
detect adulteration. The berries of V. uliginosum are among the richest
sources of flavonols – for example, myricetin, quercetin, and rutin (30-100
mg/100 g fw) compared to the berries of V. myrtillus (1-11 mg/100 g fw).63-65
In bog blueberries, delphinidin- and malvidin-glycosides predominate; however,
the anthocyanin composition reportedly varies depending on the geographical
origin of the material. The main anthocyanin in bog blueberries from Norway and
wildcrafted material from China is malvidin-3-O-glucoside, while cultivated Chinese material contains cyanidin-3-O-glucoside as the major anthocyanin.21,66-68
The range of the relative proportions of cyanidin and malvidin (calculated
either as glycosides or as aglycones after hydrolysis) is different in the
berries of V. myrtillus (26-40% and 9-15%, respectively) compared to the
berries of V. uliginosum (4-10% and 28-49%, respectively).21
Vaccinium vitis-idaea: Phytochemical
research on lingonberry has mainly focused on the phenolic composition
(anthocyanins, flavonols, phenolic acids, and proanthocyanidins). The
anthocyanin pattern in lingonberries is rather simple and consists mainly of
cyanidin-glycosides (predominantly cyanidin-3-O-galactoside).64,69,70 The flavonol composition
reportedly consists of quercetin-glycosides (quercitrin, hyperoside, and quercetin
4”-(3-hydroxy-3-methylglutaroyl)rhamnoside [HMG-rhamnoside]) and kaempferol derivatives.21,71
The presence of quercetin-HMG-rhamnoside, or the different anthocyanin
composition, has been reported to be useful in the detection of adulteration of
bilberry fruit extract with that made from lingonberry.21
Table 2: Phenolic acid, anthocyanin, and flavonol marker compounds in berries and
fruit other than bilberry and related Vaccinium
spp.*
|
Marker compound not found in bilberries
|
Source plant(s): Common name (Latin name)
|
|
Phenolic acids
|
|
|
Caffeoyltartaric acid
(syn: caftaric acid)
|
Grape (Vitis vinifera)
|
|
Coumaroyltartaric acid
|
Grape
|
|
Feruloyltartaric acid
|
Grape
|
|
Anthocyanins
|
|
|
Delphinidin-3,5-O-diglucoside
|
Pomegranate (Punica
granatum); muscadine grape (Vitis
rotundifolia)
|
|
Delphinidin-3-O-rutinoside
|
Black currant (Ribes nigrum); European
elder (Sambucus nigra)
|
|
Cyanidin-3-O-2G-glucosylrutinoside
|
Raspberry
(Rubus idaeus); Rubus hybrids; red currant
(Ribes rubrum)
|
|
Cyanidin-3-O-sophoroside-5-O-rhamnoside
|
Raspberry
|
|
Cyanidin-3-O-sambubioside-5-O-glucoside
|
American elder (Sambucus
nigra ssp. canadensis); European
elder
|
|
Cyanidin-3-O-2G-xylosylrutinoside
|
Red currant; black raspberry (Rubus
occidentalis)
|
|
Cyanidin-3,5,-O-diglucoside
|
Pomegranate; raspberry;
American elder; European elder; fox grape (Vitis labrusca); muscadine grape
|
|
Cyanidin-3-O-sophoroside
|
Black mulberry (Morus nigra); raspberry;
Rubus hybrids; red currant
|
|
Cyanidin-3-O-rutinoside
|
Black mulberry and other Morus spp.; sweet
cherry (Prunus avium); sour cherry (Prunus cerasus); European
gooseberry (Ribes uva-crispa); black currant; red currant; red-flower
currant (Ribes sanguineum); Andes berry (Rubus glaucus); Rubus hybrids; raspberry; boysenberry (Rubus loganobaccus)
|
|
Cyanidin-3-O-(6''-O-p-coumaroyl)-sambubioside-5-O-glucoside
|
American elder
|
|
Cyanidin-3-O-(6''-O-dioxalyl)-glucoside
|
Raspberry; Rubus hybrids
|
|
Petunidin-3,5-O-diglucoside
|
Muscadine grape
|
|
Petunidin-3-O-rutinoside
|
Black currant
|
|
Peonidin-3,5-O-diglucoside
|
Muscadine grape
|
|
Peonidin-3-O-rutinoside
|
European gooseberry; black currant
|
|
Pelargonidin-3,5-O-diglucoside
|
Pomegranate; muscadine
grape
|
|
Pelargonidin 3-O-rutinoside
|
Strawberry (Fragaria vesca); black mulberry and other Morus spp.; sweet cherry; Nanking
cherry (Prunus tomentosa); Andes
berry; raspberry
|
|
Pelargonidin 3-O-glucoside
|
Strawberry; black
mulberry; raspberry
|
|
Pelargonidin 3-O-(6''-O-malonyl)-glucoside
|
Strawberry
|
|
Flavonol
glycosides
|
|
|
Myricetin 3-O-rutinoside
|
Black currant
|
|
Myricetin 3-O-(6''-O-malonyl-glucoside
|
Black currant
|
|
Quercetin-3-O-rhamnosyl-(1→6)-galactoside
|
Black chokeberry (Aronia melanocarpa)
|
|
Quercetin-3-O-arabinosyl-(1→6)-glucoside
|
Black chokeberry
|
|
Quercetin 3-O-glucosyl-(1→6)-xyloside
|
Rubus hybrids
|
|
Quercetin 3-O-xylosyl-(1→6)-glucuronide
|
Rubus hybrids
|
|
Quercetin 3-O-(6''-O-malonyl)-glucoside
|
Strawberry; black currant
|
|
Isorhamnetin 3-O-rutinoside
|
Black currant; European elder; American elder
|
|
Kaempferol 3-O-rutinoside
|
Black currant; European elder; American elder
|
|
Kaempferol 3-(6''-O-malonyl)-glucoside
|
Strawberry; black currant
|
*Modified from reference 21.
8.2
Laboratory Methods
Note: Unless otherwise
noted, all methods summarized below are based on using only the fruit of bilberry
and its known adulterants.
8.2.1
HPTLC
Methods from the following sources were evaluated in this
review: Upton,12 the PhEur 8.4 monograph for bilberry extract,13
the USP 38-NF 33 Powdered Bilberry Extract
monograph,20 the CAMAG application note,72 Wagner and
Bladt,73 the PhEur 8.4 monograph for dry bilberry fruit,74
and the USP Dietary Supplements
Compendium.75
Comments: HPTLC
fingerprints are a good means to authenticate bilberry fruit extracts and
detect adulteration, although there are obvious differences among the various
published methods. The sample preparation generally consists in dissolving the
bilberry extract (or powdered dry fruit in references 72 and 74) in methanol by
shaking for 10-15 min, and subsequent filtration or centrifugation – allowing
for the preparation of a test sample using a low amount of solvent in less than
30 min. The n-butanol-acetic
acid-water (5:1:2) solvent system on silica gel plates leads to anthocyanin tailing,
which does not allow for a clear distinction of the anthocyanin pattern.73
However, the same system with cellulose plates provides better separation and
peak shapes. The chromatographic conditions described in references 12, 72, 74,
and 75 use silica gel plates and a single mobile phase consisting of formic
acid, water, and n-butanol (although
the solvent proportions in reference 74 are different than those described in 12,
72, and 75) and provide suitable conditions for bilberry authentication. With
this method, adulteration with amaranth dye at concentrations as low as 0.25%
can be detected.72 An advantage of references 12, 72, and 75 (compared
to the compendial methods in 13, 20, and 74) is the inclusion of color photographs
enabling a comparison with commercially available extracts.
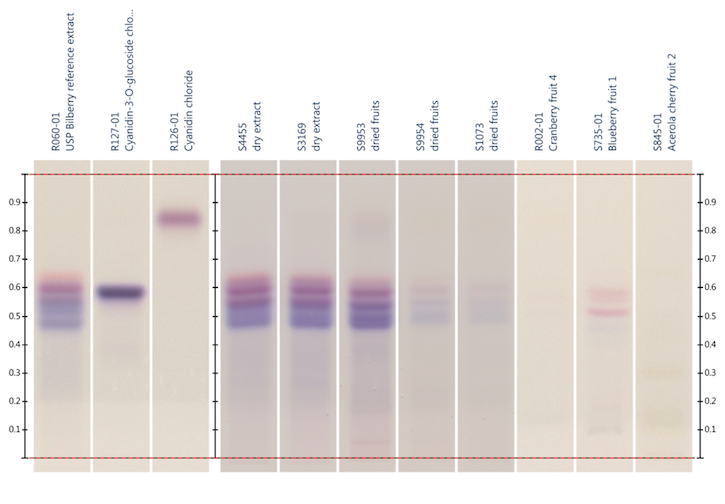
Figure 2: HPTLC analysis of bilberry fruit extract, bilberry fruit, cranberry fruit, blueberry
fruit, and acerola cherry (Malpighia sp., Malpighiaceae) fruit according to reference 74; Detection: visible
light. Lane 2: cyanidin-3-O-glucoside chloride; lane 3: cyanidin chloride. Image provided by CAMAG
(Muttenz, Switzerland)
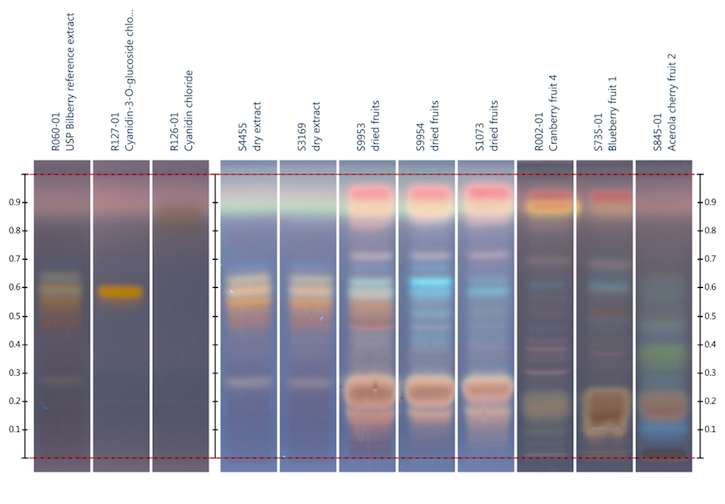
Figure 3: HPTLC
analysis of bilberry fruit extract, bilberry fruit, cranberry fruit, blueberry
fruit, and acerola cherry fruit using the stationary and mobile phase specified
in reference 74; Detection: anisaldehyde reagent, viewed under UV light at 366
nm. Lane 2: cyanidin-3-O-glucoside
chloride; lane 3: cyanidin chloride. Image provided by CAMAG (Muttenz,
Switzerland)
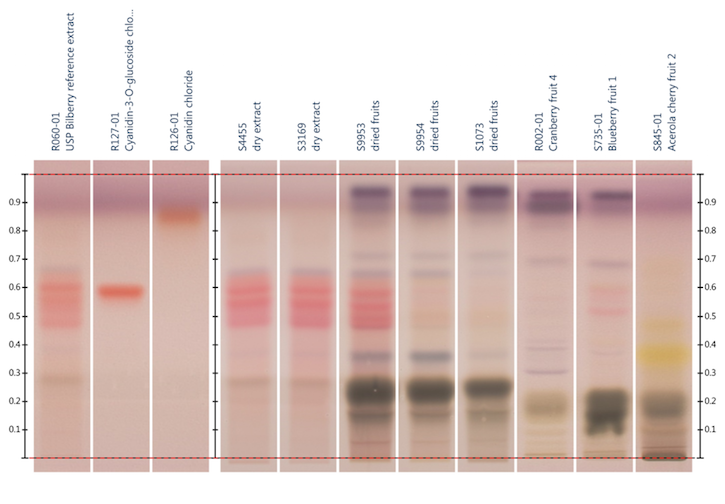
Figure
4: HPTLC analysis of bilberry fruit extract, bilberry fruit, cranberry fruit,
blueberry fruit, and acerola cherry fruit using the stationary and mobile phase
specified in reference 74; Detection: anisaldehyde reagent. Lane
2: cyanidin-3-O-glucoside chloride;
lane 3: cyanidin chloride. Image provided by CAMAG (Muttenz, Switzerland)
The USP 38-NF 3320 and PhEur 8.413 monographs for
bilberry extract use the same stationary and mobile phase in their HPTLC methods
for bilberry extract analysis, but different standard compounds: either a
reference bilberry extract20 or cyanidin-3-O-glucoside and delphinidin-3-O-glucoside13
are used. Note that in PhEur 8.6, the entire suite of bilberry monographs will
be harmonized to include only HPTLC on
silica gel plates with anhydrous formic acid, water, and n-butanol as detailed in reference 74 (Eike Reich, e-mail
communication, May 29, 2015). The chromatographic systems described in references
12, 13, 20, 72, 74, and 75 are expected to adequately distinguish bilberry
extracts from extracts of other fruit species. However, the monographs for
bilberry extract13,20 require two consecutive developing steps
before visualization and use cellulose as the stationary phase without having a
better resolution of the anthocyanin bands than when using the conditions of 12,
72 and 75, or 74. For species discrimination, the derivatization with
anisaldehyde reagent (Figures 3 and 4) is most suitable. With derivatization,
the sugar composition of bilberry fruit extract becomes visible, which can provide
additional information about the type of extract and whether or not sugar was
added to the extract.
8.2.2
HPLC and UHPLC
Methods described in the following literature were
evaluated in this review: the PhEur 8.4,13 the USP
38-NF 33,20 Lätti et al.,22 Chandra et al.,24 Govindaraghavan,32 Kalt et al.,53 Može et al.,65 Cassinese et al.,76
Penman et al.,77 Buchert et al.,78 Burdulis et al.,79
Díaz-García et al.,80 Fanali et al.,81
Gardana et al.,82 Ichiyanagi et al.,83 Jovančević et
al.,84 Müller et al.,85 Nakajima et al.,86 Obón et al.,87
Yamamoto et al.,88 and Zhang et al.89 Specific
comments on strengths and weaknesses of each of the methods are listed in Appendix
1, Table 4.
Comments: Adulteration
of bilberry extracts with natural or synthetic dyes, or anthocyanin-containing
extracts, can be detected with HPLC fingerprinting methods. For routine quality
control, quick and easy sample preparation methods are provided in the European Pharmacopoeia and the USP
38-NF 33.13,20 The
solvent of choice is usually 2% hydrochloric acid in methanol with extracts dissolved
under sonication. Note that the anthocyanin stability is limited using this
solvent. Based on the run time, separation quality, and thorough validation,
the HPLC-UV methods presented in references 13, 20, 32, and 76 appear to be the
optimal choices.
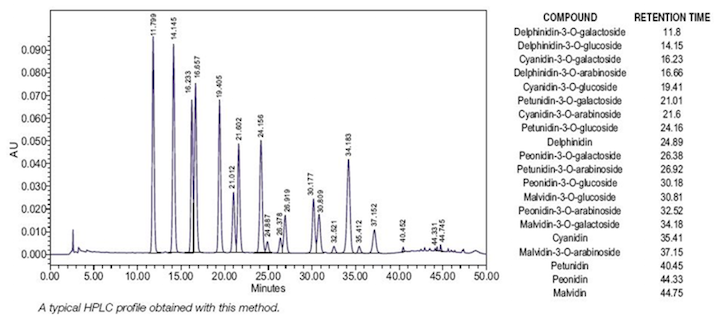
Figure
5. HPLC-UV chromatogram of an authentic bilberry extract analyzed according to
the conditions outlined in the European
Pharmacopoeia;13 Image provided by Indena S.p.A. (Milan, Italy).
The USP monograph has
additional features to authenticate bilberry: delphinidin-3-O-galactoside and delphinidin-3-O-glucoside should be the largest peaks;
the cyanidin-3-O-galactoside,
delphinidin-3-O-arabinoside, and
cyanidin-3-O-glucoside peaks should
be of similar size; and the size of each of the remaining anthocyanin peaks in
the chromatogram should be smaller than the cyanidin-3-O-glucoside peak. This could be problematic since some authentic
bilberry fruit samples (although authentication methods were not detailed) were
found to contain more malvidin-3-O-glucoside
than cyanidin-3-O-glucoside. In this
situation, some authentic bilberry fruit extracts could be rejected if the
specifications outlined in the USP monograph were to be followed.32 If
run time is critical, the conditions described by Yamamoto et al.88
are a good choice since they provide a similar separation efficiency in a 20-min
run as the HPLC methods do in 50 min.13,20,32,76 It should be noted
that this requires UHPLC instrumentation, which operates under higher pressure
than standard HPLC equipment. Validation and system suitability parameters are
lacking for the published UHPLC method.
8.2.3
UV/Vis Spectrophotometry
Methods described in the following literature were
evaluated in this review: Upton,12 the Institute for Nutraceutical
Advancement,90 the PhEur 6.0,91
and AOAC International.92
Comments: While
all these methods have the advantage of being simple and quick, and can be performed with relatively affordable instrumentation, their use to detect
adulteration of bilberry extracts is limited. The methods described in references
12 and 91 are basically the same (minor differences exist in the sample
preparation) and may allow the detection of adulteration with charcoal, but
other adulterants will absorb at the test wavelength (528 nm) and may lead to
erroneously high values for anthocyanin content. The INA method90
and the official AOAC method92 use pH-dependent differences in
absorption (anthocyanins exist as the intensely colored oxonium or flavylium
ions at pH 1.0, whereas at pH 4.5, they occur as colorless carbinols) at 520 nm
to calculate anthocyanin contents. This method has been shown to detect
adulteration with synthetic dyes, but it is not capable of identifying
anthocyanin-based adulterants from other natural sources. Therefore, UV/Vis
spectrometry is inadequate as a means to detect adulteration of bilberry
extracts.
Table
3. Comparison among the different approaches to authenticate bilberry.
|
Method
|
Applicable to
|
Pro
|
Contra
|
|
Macroscopic
|
- Unprocessed plant parts
|
Quick
Inexpensive
No solvents required
|
No
automation/statistics
Outcome
relies on analyst’s expertise
Challenging
for cut and sifted material
|
|
Microscopic
|
- Unprocessed plant parts
|
Quick
Inexpensive
|
No
automation/statistics
Outcome
relies on analyst’s expertise
Difficult
or impossible to distinguish closely related species
|
|
Genetic
|
- Unprocessed plant parts
- Cut and sifted
- Powdered
|
Able to
distinguish closely related species
|
Labor-intensive
sample preparation and analysis
Expensive
equipment
Unable to
differentiate plant parts
Cannot
detect dyed or pre-extracted materials
May not be
applicable to highly processed materialsa
|
|
HPTLC
|
- Cut and sifted
- Powdered
- Extracts
|
Quick
Basic
systems affordable for smaller labs
|
No
statistics
High-end
equipment expensive
Detection
of adulteration challenging when related Vaccinium
species are mixed
Need for
standard compounds
|
|
HPLC-UV
|
- Cut and sifted
- Powdered
- Extracts
|
Standard
equipment in many laboratories
Ideal for
compounds with strong chromophore (e.g., phenolic acids)
|
Equipment
expensive
Mostly
quantitative (less specific than HPLC-UV/MS)
Detection
of adulteration challenging when related Vaccinium
species are mixed
Need for
standard compounds
|
|
HPLC-UV/MS
|
- Cut and sifted
- Powdered
- Extracts
|
Qualitative
and quantitative
State-of-the-art
statistical evaluation possible
|
Equipment
expensive
Detection
of adulteration challenging when related Vaccinium
species are mixed
|
|
UV/Vis
|
- Cut and sifted
- Powdered
- Extracts
|
Quick
Inexpensive
Method
based on absorption at different pHs is able to detect adulteration with
synthetic dyes
|
Unable to
detect adulteration in most cases
No
statistics
|
|
FT-NIR
|
- Powdered
- Extracts
|
Quick
Inexpensive
State-of-the-art
statistical evaluation
Result
does not rely on analyst’s expertise
|
Need to
build-up reference library
No data
on the ability to detect adulteration (except blueberry93)
|
aHeat,
UV light, radiation, and mutagenic chemicals (e.g., polyaromatic hydrocarbons)
can damage DNA. Extracts made using lipophilic solvents will not contain DNA.
9.
Conclusion
Based on an evaluation of
published methods, the most effective approach to detect adulteration of
bilberry fruit extracts may be based on the evaluation of a phytochemical
fingerprint. Several published HPTLC methods have shown their ability to
distinguish bilberry fruit extract and its major adulterants.12,13,20,72,74,75
The HPTLC methods of choice for detection of bilberry fruit extract substitution
are described in the European Pharmacopoeia74
and in references 12, 72, and 75 using the anisaldehyde reagent for detection. Admixtures
of other anthocyanin-containing extracts can also be detected in many instances
using HPTLC, a possible exception being if bilberry and blueberry extracts are
mixed.
HPLC has the added benefit
that peak size can be easily evaluated, which is a helpful tool for the
detection of bilberry and adulterant extract mixtures. Several authors and
compendia propose comparable methods,13,20,32,76 which can be
recommended based on ease of use, extensive validation, and proven ability to
detect a wide array of adulterants. Care should be taken when using the
additional criteria established in the USP for authentication, since the
natural variability in bilberry may possibly lead to rejection of extracts made
from authentic material from certain geographical locations.32
Note: A number of identity tests for bilberry extracts
are offered by third-party analytical laboratories. According to input from five
contract laboratories, the main testing methods are HPTLC and HPLC-UV. Additional
testing methods (FT-NIR and HPLC-MS) are offered by some laboratories, or can be
developed upon request.
10. References
- Foster S,
Blumenthal M. The adulteration of commercial bilberry
extracts. HerbalGram. 2012;(96):64-73. Available at: http://cms.herbalgram.org/herbalgram/issue96/hg96-feat-bilberry.html.
Accessed August 12, 2015.
- McGuffin M,
Kartesz JT, Leung AY, Tucker AO. Herbs of
Commerce. 2nd ed. Silver Spring, MD: American Herbal Products
Association; 2000.
- The Plant
List. Version 1.1 (September 2013). Available at: http://www.theplantlist.org. Accessed
July 9, 2015.
- United States
Department of Agriculture (USDA), Agricultural Research Service (ARS), National
Genetic Resources Program. Germplasm Resources Information Network (GRIN)
Online Database. Beltsville, MD: National Germplasm Resources Laboratory. Available at: http://www.ars-grin.gov. Accessed July 9, 2015.
- Ritchie JC.
Biological flora of the British Isles: Vaccinium
myrtillus L. J Ecol.
1956;44(1):291-299.
- Tropicos.org.
Missouri Botanical Garden. Available at: http://www.tropicos.org.
Accessed July 9, 2015.
- USDA PLANTS
Database. Available at: http://plants.usda.gov.
Accessed July 9, 2015.
- Health Canada
Natural Health Products Ingredients Database. Available at: http://webprod.hc-sc.gc.ca/nhpid-bdipsn/ingredsReq.do?srchRchTxt=Vaccinium&srchRchRole=-1&mthd=slct&ind=2&lang=eng.
Accessed July 9, 2015.
- Flora of China. eFloras.org website. Available at: http://www.efloras.org. Accessed July 9, 2015.
- Gruenwald J, Brendler T, Jaenicke C, et al. (eds). PDR for Herbal Medicines. 2nd ed. Montvale, NJ: Medical
Economics Company, Inc.; 2000.
- Pace R,
Morazzoni P, Appendino G. Omne
ignotum pro magnifico:
Getting bilberry out of the adulteration swamp. Talk presented at: 9th
Oxford International Conference on the Science of Botanicals; April 14, 2010;
Oxford, MS.
- Upton R, ed. American
Herbal Pharmacopoeia and Therapeutic Compendium: Bilberry Fruit Vaccinium myrtillus L.: Standards of
Analysis, Quality Control, and Therapeutics. Scotts Valley, CA:
American Herbal Pharmacopoeia; 2001.
- The European Directorate for the Quality of Medicines & HealthCare. European Pharmacopoeia (EP 8.4). Myrtilli
fructus recentis extractum siccum raffinatum et normatum. Strasbourg,
France: Council of Europe; 2014.
- Frohne D.
Myrtilli fructus siccus – Dried bilberry fruit. In: Wichtl M, ed. Brinckmann JA, Lindenmaier MP, trans. Herbal Drugs and Phytopharmaceuticals. 3rd
ed. Stuttgart, Germany: Medpharm Scientific Publishers; 2004: 411-413.
- Filippini R,
Piovan A, Caniato R. Substitution of Vaccinium myrtillus L. for Aronia melanocarpa (Michx.) Elliott in a
commercial batch. Plant Biosyst.
2011;145(1):175-181. Abstract available at: http://www.tandfonline.com/doi/abs/10.1080/11263504.2010.543790.
Accessed August 12, 2015.
- Upton R,
Graff A, Jolliffe G, Länger R, Williamson E, eds. American Herbal Pharmacopoeia: Botanical
Pharmacognosy—Microscopic Characterization of Botanical Medicines. Boca Raton, FL: CRC Press; 2011.
- Eschrich W. Pulver-Atlas der Drogen
der Deutschsprachigen Arzneibücher. 7th ed. Stuttgart, Germany:
Deutscher Apotheker Verlag; 1999.
- Villani TS, Patel H, Zheng J, Koroch AR, Simon JE. Microscopy for quality assessment of bilberry
fruit (Vaccinium myrtillus L.).
Journal of Medicinally Active Plants.
2015;4(1-2):8-15. Available at: http://scholarworks.umass.edu/jmap/vol4/iss1/3/.
Accessed August 12, 2015.
- Jaakola L,
Suokas M, Häggman H. Novel approaches based on DNA barcoding and
high-resolution melting of amplicons for authenticity analyses of berry
species. Food Chem.
2010;123(2):494-500. Abstract available at: http://www.sciencedirect.com/science/article/pii/S0308814610005261.
Accessed August 12, 2015.
- United
States Pharmacopeia and National Formulary (USP 38-NF 33).
Powdered Bilberry Extract. Rockville, MD: United States Pharmacopeial
Convention; 2014.
- Primetta A. Phenolic compounds in the berries of the
selected Vaccinium species: the potential for authenticity analyses.
PhD thesis. Kuopio, Finland: University of Eastern Finland, Dissertations in
Forestry and Natural Sciences; 2014. Available at: http://epublications.uef.fi/pub/urn_isbn_978-952-61-1360-9/urn_isbn_978-952-61-1360-9.pdf.
Accessed July 9, 2015.
- Lätti AK,
Riihinen KR, Kainulainen PS. Analysis of anthocyanin variation in wild
populations of bilberry (Vaccinium myrtillus L.) in Finland. J
Agric Food Chem. 2008;56(1):190-196.
Abstract available at: http://pubs.acs.org/doi/abs/10.1021/jf072857m.
Accessed August 12, 2015.
- Kulling SE,
Rawel HM. Chokeberry (Aronia melanocarpa) – a
review on the characteristic components and potential health effects. Planta Med. 2008;74(13):1625-1634.
Available
at: https://www.thieme-connect.com/products/ejournals/html/10.1055/s-0028-1088306.
Accessed August 12, 2015.
- Chandra A, Rana J, Li Y. Separation, identification,
quantification, and method validation of anthocyanins in botanical supplement
raw materials by HPLC and HPLC-MS. J Agric Food Chem. 2001;49(8):3515-3521. Abstract
available at: http://pubs.acs.org/doi/abs/10.1021/jf010389p.
Accessed August 12, 2015.
- Strigl AW, Leitner E, Pfannhauser W. Qualitative und quantitative analyse
der anthocyane in schwarzen apfelbeeren (Aronia melanocarpa Michx Ell)
mittels TLC, HPLC and UV/VIS-spectrometrie. Z
Lebensm Unters Forsch. 1995;201(3):266-268. Abstract available at: http://link.springer.com/article/10.1007%2FBF01193001.
Accessed August 13, 2015.
- Slimestad R, Torskangerpoll K, Nateland HS, Johannessen T, Giske NH. Flavonoids from black chokeberries, Aronia
melanocarpa. J Food Compost Anal. 2005;18(1):61-68. Abstract available at: http://www.sciencedirect.com/science/article/pii/S0889157503001789.
Accessed August 13, 2015.
- Chajuss D.
Soy phytochemical composition. US Patent Application Number US20020119208 A1.
Available at: http://appft.uspto.gov/netacgi/nph-Parser?Sect1=PTO2&Sect2=HITOFF&p=1&u=%2Fnetahtml%2FPTO%2Fsearch-bool.html&r=3&f=G&l=50&co1=AND&d=PG01&s1=Chajuss.IN.&OS=IN/Chajuss&RS=IN/Chajuss.
Accessed July 9, 2015.
- Ha TJ, Lee JH, Shin S-O, et al. Changes in
anthocyanin and isoflavone concentrations in black seed-coated soybean at
different planting locations. J Crop Sci Biotechnol. 2009;12(2):79-86. Abstract available
at: http://link.springer.com/article/10.1007%2Fs12892-009-0093-9.
Accessed August 13, 2015.
- Wang C, Sherrard M, Pagadala S, Wixon R, Scott RA. Isoflavone content among maturity group 0 to II soybeans. J Am Oil Chem Soc. 2000;77(5):483-487. Abstract
available at: http://link.springer.com/article/10.1007%2Fs11746-000-0077-6.
Accessed August 13, 2015.
- Slavin M, Kenworthy W, Yu L. Antioxidant properties, phytochemical composition,
and antiproliferative activity of Maryland-grown soybeans with colored seed
coats. J Agric Food Chem. 2009;57(23):11174-11185. Abstract available at: http://pubs.acs.org/doi/abs/10.1021/jf902609n.
Accessed August 13, 2015.
- Fukuda I,
Tsutsui M, Yoshida T, Toda T, Tsuda T, Ashida H. Oral toxicological studies of
black soybean (Glycine max) hull
extract: Acute studies in rats and mice, and chronic studies in mice. Food Chem Toxicol. 2011;49(12):3272-3278. Abstract available at: http://www.sciencedirect.com/science/article/pii/S0278691511004662.
Accessed August 13, 2015.
- Govindaraghavan
S. Pharmacopeial HPLC identification methods are not sufficient to detect
adulterations in commercial bilberry (Vaccinium
myrtillus) extracts. Anthocyanin profile provides additional clues. Fitoterapia. 2014;99:124-138. Abstract
available at: http://www.sciencedirect.com/science/article/pii/S0367326X1400255X.
Accessed August 13, 2015.
- Lee JH, Kang
NS, Shin S-O, et al. Characterisation of anthocyanins in the black soybean (Glycine max L.) by HPLC-DAD-ESI/MS
analysis. Food Chem.
2009;112(1):226-231. Abstract available at: http://www.sciencedirect.com/science/article/pii/S0308814608006183.
Accessed August 13, 2015.
- Wu T, Qi X,
Liu Y, et al. Dietary supplementation with purified mulberry (Morus australis Poir) anthocyanins
suppresses body weight gain in high-fat diet fed C57BL/6 mice. Food Chem. 2013;141(1):482-487. Abstract
available at: http://www.sciencedirect.com/science/article/pii/S030881461300349X.
Accessed August 13, 2015.
- Song W, Wang
H-J, Bucheli P, Zhang P-F, Wei D-Z, Lu Y-H. Phytochemical profiles of different
mulberry (Morus sp.) species from China. J Agric Food Chem. 2009;57(19):9133-9140. Abstract available at: http://pubs.acs.org/doi/abs/10.1021/jf9022228.
Accessed August 13, 2015.
- Koyuncu F. Organic acid composition of native black mulberry fruit.
Chemistry of Natural Compounds.
2004;40(4):367-369. Abstract
available at: http://link.springer.com/article/10.1023%2FB%3ACONC.0000048249.44206.e2.
Accessed August 13, 2015.
- Kamiloglu S, Serali O, Unal N, Capanoglu E. Antioxidant activity and
polyphenol composition of black mulberry (Morus
nigra L.) products. Journal of Berry
Research. 2013;3(1):41-51.
Available at: http://content.iospress.com/articles/journal-of-berry-research/jbr045.
Accessed August 13, 2015.
- Veberic R,
Slatnar A, Bizjak J, Stampar F, Mikulic-Petkovsek M. Anthocyanin composition of different wild and cultivated berry species. LWT
- Food Science and Technology. 2015;60(1):509-517. Abstract
available at: http://www.sciencedirect.com/science/article/pii/S0023643814005416.
Accessed August 13, 2015.
- Pawlowska AM, Oleszek W, Braca A. Quali-quantitative analyses of
flavonoids of Morus nigra L. and Morus
alba L. (Moraceae) fruits. J Agric Food Chem. 2008;56(9):3377-3380. Abstract available at: http://pubs.acs.org/doi/abs/10.1021/jf703709r.
Accessed August 13, 2015.
- Friedman M. Rice
brans, rice bran oils, and rice hulls: composition, food and industrial uses,
and bioactivities in humans, animals, and cells. J Agric Food Chem. 2013;61(45):10626-10641. Abstract available at: http://pubs.acs.org/doi/abs/10.1021/jf403635v.
Accessed August 13, 2015.
- Goufo P,
Trindade H. Rice antioxidants: phenolic acids, flavonoids, anthocyanins,
proanthocyanidins, tocopherols, tocotrienols, γ-oryzanol, and phytic acid. Food Sci Nutr. 2014;2(2):75-104.
Available at: http://onlinelibrary.wiley.com/doi/10.1002/fsn3.86/abstract.
Accessed August 13, 2015.
- Salanti A,
Zoia L, Orlandi M, Zanini F, Elegir G. Structural characterization and
antioxidant activity evaluation of lignins from rice husk. J Agric Food Chem. 2010;58(18):10049-10055. Abstract available at: http://pubs.acs.org/doi/abs/10.1021/jf102188k.
Accessed August 13, 2015.
- Ballistreri
G, Continella A, Gentile
A, Amenta M, Fabroni S, Rapisarda P. Fruit quality
and bioactive compounds relevant to human health of sweet cherry (Prunus avium L.) cultivars grown in
Italy. Food Chem.
2013;140(4):630-638. Abstract available at: http://www.sciencedirect.com/science/article/pii/S0308814612017517.
Accessed August 13, 2015.
- Cao
J, Jiang Q, Lin J, Li X, Sun C, Chen K. Physicochemical characterisation of
four cherry species (Prunus spp.)
grown in China. Food Chem. 2015;173:855-863. Abstract available at: http://www.sciencedirect.com/science/article/pii/S0308814614016574.
Accessed August 13, 2015.
- Kelebek
H, Selli S. Evaluation of
chemical constituents and antioxidant activity of sweet cherry (Prunus avium L.) cultivars. Int J Food Sci Technol.
2011;46(12):2530-2537. Abstract available at: http://onlinelibrary.wiley.com/doi/10.1111/j.1365-2621.2011.02777.x/abstract.
Accessed August 13, 2015.
- Vagiri M. Black currant (Ribes nigrum L.) – an
insight into the crop. PhD thesis. Balsgård, Sweden: Swedish University
of Agricultural Sciences; 2012. Available at: http://pub.epsilon.slu.se/8586/17/vagiri_m_120206_small.pdf.
Accessed July 9, 2015.
- Wu X, Gu L, Prior RL, McKay S. Characterization of anthocyanins and
proanthocyanidins in some cultivars of Ribes,
Aronia, and Sambucus and their antioxidant capacity. J
Agric Food Chem. 2004;52(26):7846-7856. Abstract
available at: http://pubs.acs.org/doi/abs/10.1021/jf0486850.
Accessed August 13, 2015.
- Rubinskiene M, Jasutiene I, Venskutonis PR, Viskelis P. HPLC
determination of the composition and stability of blackcurrant anthocyanins. J
Chromatogr Sci.
2005;43(9):478-482. Available at: http://chromsci.oxfordjournals.org/content/43/9/478.abstract.
Accessed August 13, 2015.
- Sójka M,
Guyot S, Kołodziejczyk K, Król B, Baron A. Composition and properties of purified
phenolics preparations obtained from an
extract of industrial blackcurrant (Ribes
nigrum L.) pomace. J Hortic Sci
Biotechnol. 2009;ISAFRUIT Special Issue:100-106. Available at: http://www.jhortscib.com/isafruit/isa_pp100_106.pdf.
Accessed August 13, 2015.
- Lee J,
Dossett M, Finn CE. Rubus fruit
phenolic research: The good, the bad, and the confusing. Food Chem. 2012;130(4):785-796. Abstract available at: http://www.sciencedirect.com/science/article/pii/S0308814611011496.
Accessed August 13, 2015.
- Kaume L,
Howard LR, Devareddy L. The blackberry fruit: a review on its composition and
chemistry, metabolism and bioavailability, and health benefits. J Agric Food Chem. 2012;60(23):5716-5727. Abstract
available at: http://pubs.acs.org/doi/abs/10.1021/jf203318p.
Accessed August 13, 2015.
- Lee J, Finn CE. Anthocyanins and other polyphenolics in American elderberry (Sambucus canadensis) and European
elderberry (S. nigra)
cultivars. J Sci Food Agric.
2007;87(14):2665-2675. Abstract available at: http://onlinelibrary.wiley.com/doi/10.1002/jsfa.3029/abstract.
Accessed August 13, 2015.
- Kalt W,
McDonald JE, Ricker RD, Lu X. Anthocyanin
content and profile within and among blueberry species. Can J Plant Sci. 1999;79(4):617-623.
Available at: http://pubs.aic.ca/doi/abs/10.4141/P99-009.
Accessed August 13, 2015.
- Wu X, Prior
RL. Systematic identification and characterization of anthocyanins by
HPLC-ESI-MS/MS in common foods in the United States: fruits and berries. J Agric Food Chem. 2005;53(7):2589-2599. Abstract
available at: http://pubs.acs.org/doi/abs/10.1021/jf048068b.
Accessed August 13, 2015.
- Barnes JS,
Nguyen HP, Shen S, Schug KA. General method for extraction of blueberry
anthocyanins and identification using high performance liquid
chromatography–electrospray ionization-ion trap-time of flight-mass
spectrometry. J Chromatogr A.
2009;1216(23):4728-4735. Abstract available at: http://www.sciencedirect.com/science/article/pii/S0021967309005573.
Accessed August 13, 2015.
- Giacomelli L, Appendino G, Franceschi F, Togni S, Pace R. Omne
ignotum pro magnifico:
characterization of commercial bilberry extracts to fight adulteration. Eur Rev Med Pharmacol Sci.
2014;18(24):3948-3953. Available at: http://www.europeanreview.org/article/8275.
Accessed August 13, 2015.
- Häkkinen S, Heinonen M, Kärenlampi S, Mykkänen H,
Ruuskanen J, Törrönen R. Screening of
selected flavonoids and phenolic acids in 19 berries. Food Res Int. 1999;32(5):345-353. Abstract available at: http://www.sciencedirect.com/science/article/pii/S0963996999000952.
Accessed August 13, 2015.
- Riihinen K. Phenolic compounds in berries. PhD thesis. Kuopio, Finland: Kuopio
University Publications C. Natural and Environmental Sciences; 2005. Available at:
http://wanda.uef.fi/uku-vaitokset/vaitokset/2005/isbn951-27-0345-9.pdf.
Accessed July 9, 2015.
- Adamczak A,
Buchwald W, Kozłowski J. Variation in the
content of flavonols and main organic acids in the fruit of European cranberry
(Oxycoccus palustris Pers.) growing
in peatlands of North-Western Poland. Herba Polonica. 2011;57(4):5-15. Available at: http://www.researchgate.net/publication/262419204_Variation_in_the_content_of_flavonols_and_main_organic_acids_in_the_fruit_of_European_cranberry_(Oxycoccus_palustris_Pers.)_growing_in_peatlands_of_north-western_Poland.
Accessed August 13, 2015.
- Jensen HD,
Krogfelt KA, Cornett C, Hansen SH, Christensen SB. Hydrophilic carboxylic acids
and iridoid glycosides in the juice of American and European cranberries (Vaccinium macrocarpon and V. oxycoccos), lingonberries (V. vitis-idaea), and blueberries (V. myrtillus). J Agric Food Chem. 2002;50(23):6871-6874.
Abstract available at: http://pubs.acs.org/doi/abs/10.1021/jf0205110.
Accessed August 13, 2015.
- Brown PN,
Turi CE, Shipley PR, Murch SJ. Comparisons of large (Vaccinium macrocarpon Ait.) and small (Vaccinium oxycoccos L., Vaccinium
vitis-idaea L.) cranberry in British Columbia by phytochemical
determination, antioxidant potential, and metabolomic profiling with
chemometric analysis. Planta
Med. 2012;78(6):630-640. Available at: https://www.thieme-connect.com/DOI/DOI?10.1055/s-0031-1298239.
Accessed August 13, 2015.
- Huopalahti R, Järvenpää EP, Katina K. A novel solid-phase extraction-HPLC method for the analysis of
anthocyanin and organic acid composition of Finnish cranberry. J Liq Chromatogr
Relat Technol. 2000;23(17):2695-2701. Abstract
available at: http://www.tandfonline.com/doi/abs/10.1081/JLC-100101827.
Accessed August 13, 2015.
- Häkkinen SH,
Törrönen AR.
Content
of flavonols and selected phenolic acids in strawberries and Vaccinium
species: influence of cultivar, cultivation site and technique. Food Res Int. 2000;33(6):517-524.
Abstract available at: http://www.sciencedirect.com/science/article/pii/S0963996900000867.
Accessed August 13, 2015.
- Määttä-Riihinen
KR, Kamal-Eldin A, Mattila PH, González-Paramás AM, Törrönen AR. Distribution
and contents of phenolic compounds in eighteen Scandinavian berry species. J Agric Food Chem.
2004;52(14):4477-4486. Abstract available at: http://pubs.acs.org/doi/abs/10.1021/jf049595y.
Accessed August 13, 2015.
- Može Š, Polak
T, Gašperlin L, et al. Phenolics in Slovenian bilberries (Vaccinium
myrtillus L.) and blueberries (Vaccinium corymbosum L.). J Agric Food Chem.
2011;59(13):6998-7004. Abstract available at: http://pubs.acs.org/doi/abs/10.1021/jf200765n.
Accessed August 13, 2015.
- Andersen ØM.
Anthocyanins in fruits of Vaccinium
uhginosum [sic] L. (bog
whortleberry). J Food Sci. 1987;52(3):665-666.
Abstract available at: http://onlinelibrary.wiley.com/doi/10.1111/j.1365-2621.1987.tb06698.x/abstract.
Accessed August 13, 2015.
- Li R, Wang P,
Guo Q-Q, Wang Z-Y. Anthocyanin composition and content of the Vaccinium uliginosum berry. Food Chem. 2011;125(1):116-120. Abstract
available at: http://www.sciencedirect.com/science/article/pii/S0308814610010496.
Accessed August 13, 2015.
- Liu J, Zhang
W, Jing H, Popovich DG. Bog bilberry (Vaccinium
uliginosum L.) extract reduces cultured Hep-G2, Caco-2, and 3T3-L1 cell
viability, affects cell cycle progression, and has variable effects on membrane
permeability. J Food Sci.
2010;75(3):H103-H107. Abstract available at: http://onlinelibrary.wiley.com/doi/10.1111/j.1750-3841.2010.01546.x/abstract.
Accessed August 13, 2015.
- Lätti AK,
Riihinen KR, Jaakola L. Phenolic compounds in berries and flowers of a natural
hybrid between bilberry and lingonberry (Vaccinium
× intermedium Ruthe). Phytochemistry. 2011;72(8):810-815.
Abstract available at: http://www.sciencedirect.com/science/article/pii/S0031942211001014.
Accessed August 13, 2015.
- Zheng W, Wang
SY. Oxygen radical absorbing capacity of phenolics in blueberries, cranberries,
chokeberries, and lingonberries. J Agric
Food Chem. 2003;51(2):502-509. Abstract available at: http://pubs.acs.org/doi/abs/10.1021/jf020728u.
Accessed August 13, 2015.
- Ek S, Kartimo
H, Mattila S, Tolonen A. Characterization of phenolic compounds from
lingonberry (Vaccinium vitis-idaea). J
Agric Food Chem. 2006;54(26):9834-9842. Abstract available at: http://pubs.acs.org/doi/abs/10.1021/jf0623687.
Accessed August 13, 2015.
- CAMAG
Application Note F-35. HPTLC detection of the azo dye amaranth as an adulterant
of bilberry extract. Muttenz, Switzerland: CAMAG Laboratory; 2007. Available
at: http://www.camag.com/en/tlc_hptlc/camag_laboratory/methods.cfm.
Accessed July 9, 2015.
- Wagner H, Bladt S. Plant Drug
Analysis: A Thin Layer Chromatography Atlas. 2nd ed. Berlin,
Germany: Springer-Verlag; 1996:288-289.
- The European Directorate for the Quality of Medicines & HealthCare. European Pharmacopoeia (EP 8.4). Myrtilli
fructus siccus. Strasbourg, France: Council of Europe; 2014.
- USP
Dietary Supplements Compendium.
Dry Bilberry Extract. Rockville, MD: United States Pharmacopeial Convention;
2012.
- Cassinese C,
de Combarieu E, Falzoni M, Fuzzati N, Pace R, Sardone N. New liquid
chromatography method with ultraviolet detection for analysis of anthocyanins
and anthocyanidins in Vaccinium myrtillus
fruit dry extracts and commercial preparations. J AOAC Int. 2007;90(4):911-919. Abstract available at: http://www.ingentaconnect.com/content/aoac/jaoac/2007/00000090/00000004/art00004.
Accessed August 13, 2015.
- Penman KG,
Halstead CW, Matthias A, et al. Bilberry
adulteration using the food dye amaranth. J Agric Food Chem. 2006;54(19):7378-7382.
Abstract available at: http://pubs.acs.org/doi/abs/10.1021/jf061387d.
Accessed August 13, 2015.
- Buchert J,
Koponen JM, Suutarinen M, et al. Effect of enzyme-aided pressing on anthocyanin
yield and profiles in bilberry and blackcurrant juices. J Sci Food Agric. 2005;85(15):2548-2556. Abstract available at: http://onlinelibrary.wiley.com/doi/10.1002/jsfa.2284/abstract.
Accessed August 13, 2015.
- Burdulis D, Ivanauskas L,
Dirse V, Kazlauskas S, Razukas A. Study
of diversity of anthocyanin composition in bilberry (Vaccinium myrtillus L.) fruits. Medicina (Kaunas). 2007;43(12):971-977. Available at: http://medicina.lsmuni.lt/med/0712/0712-09e.htm.
Accessed August 13, 2015.
- Díaz-García MC, Obón JM, Castellar MR,
Collado J, Alacid M. Quantification by
UHPLC of total individual polyphenols in fruit juices. Food
Chem.
2013;138(2-3):938-949. Abstract available at: http://www.sciencedirect.com/science/article/pii/S0308814612017931.
Accessed August 13, 2015.
- Fanali C, Dugo L,
D'Orazio G, et al. Analysis of
anthocyanins in commercial fruit juices by using nano-liquid
chromatography-electrospray-mass spectrometry
and high-performance liquid chromatography with UV-vis detector. J Sep Sci. 2011;34(2):150-159. Abstract
available at: http://onlinelibrary.wiley.com/doi/10.1002/jssc.201000665/abstract.
Accessed August 13, 2015.
- Gardana C,
Ciappellano S, Marinoni L, Fachechi C, Simonetti P. Bilberry adulteration:
identification and chemical profiling of anthocyanins by different analytical
methods. J Agric Food Chem.
2014;62(45):10998-11004. Abstract available at: http://pubs.acs.org/doi/abs/10.1021/jf504078v.
Accessed August 13, 2015.
- Ichiyanagi T, Hatano Y,
Matsugo S, Konishi T. Structural
dependence of HPLC separation pattern of anthocyanins from bilberry (Vaccinium
myrtillus L.). Chem Pharm Bull (Tokyo). 2004;52(5):628-630. Available at: https://www.jstage.jst.go.jp/article/cpb/52/5/52_5_628/_article.
Accessed August 13, 2015.
- Jovančević
M, Balijagić J, Menković N, et al. Analysis of
phenolic compounds in wild populations of bilberry (Vaccinium myrtillus L.) from Montenegro. J Med Plant Res. 2011;5(6):910-914. Available
at: http://www.academicjournals.org/journal/JMPR/article-abstract/7E792C119538.
Accessed August 13, 2015.
- Müller D, Schantz M,
Richling E. High performance liquid chromatography
analysis of anthocyanins in bilberries (Vaccinium myrtillus
L.), blueberries (Vaccinium
corymbosum L.), and corresponding juices. J Food Sci. 2012;77(4):C340-C345. Abstract
available at: http://onlinelibrary.wiley.com/doi/10.1111/j.1750-3841.2011.02605.x/abstract.
Accessed August 13, 2015.
- Nakajima JI, Tanaka I,
Seo S, Yamazaki M, Saito K. LC/PDA/ESI-MS profiling and
radical scavenging activity of anthocyanins in various berries. J Biomed Biotechnol. 2004;2004(5):241-247.
Available at: http://www.hindawi.com/journals/bmri/2004/469084/abs/.
Accessed August 13, 2015.
- Obón JM, Díaz-García MC, Castellar MR. Red fruit juice quality and authenticity
control by HPLC. J Food Compost
Anal. 2011;24(6):760-771. Abstract available at: http://www.sciencedirect.com/science/article/pii/S088915751100086X.
Accessed August 13, 2015.
- Yamamoto M,
Yamaura K, Ishiwatari M, et al. Degradation index for quality evaluation of
commercial dietary supplements of bilberry extract. J Food Sci. 2013;78(3):S477-S483. Abstract available at: http://onlinelibrary.wiley.com/doi/10.1111/1750-3841.12043/abstract.
Accessed August 13, 2015.
- Zhang Z, Kou
X, Fugal K, McLaughlin J. Comparison of HPLC methods for determination of
anthocyanins and anthocyanidins in bilberry extracts. J Agric Food Chem. 2004;52(4):688-691. Abstract available at: http://pubs.acs.org/doi/abs/10.1021/jf034596w.
Accessed August 13, 2015.
- Institute for
Nutraceutical Advancement. Anthocyanin content in bilberry by pH-differential
spectrophotometry. INA Method 116.000.
- The European Directorate for the Quality of Medicines & HealthCare. European
Pharmacopoeia (EP 6.0).
Myrtilli fructus recens. Strasbourg, France: Council of Europe;
2008:1307-1308.
- Lee J, Durst R, Wrolstad R. AOAC Official Method 2005.02. Total monomeric
anthocyanin pigment content of fruit juices, beverages, natural colorants, and
wines by the pH differential method. In: Horowitz H, ed. Official Methods of Analysis of AOAC International. Washington, DC:
AOAC International; 2006.
- Jaksch F. ChromaDex’s ComplyID FT-NIR Identification Program. Talk presented at: 2014 AHPA Botanical Congress: Tools and Methods for Botanical Identification; October 10, 2014; Las Vegas, NV.
- LaBudde
RA, Harnly JM. Probability of identification: a statistical model for the
validation of qualitative botanical identification methods. J AOAC Int. 2012;95(1):273-285.
Available at: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3620024/pdf/nihms450355.pdf. Accessed July 9, 2015.
Appendix
1
Table
4: Comments on the published HPLC methods to authenticate bilberry extracts and
detect adulteration.
|
Reference
|
Comments
|
|
EP 8.4,13 USP 38-NF 33,20 Govindaraghavan,32 Cassinese76
|
The validated
HPLC-UV fingerprinting methods detailed by the USP20 and the European Pharmacopoeia13 have
only minor differences (the difference being the concentration of formic acid
in the mobile phase). Both provide excellent separations with good peak
shapes over the 50-min run time.a Data from industry11 have
shown that these methods are capable of detecting adulteration with a variety
of anthocyanidin-containing extracts and amaranth dye.
|
|
Lätti22
|
This fingerprint method shows a good separation of anthocyanins and acceptable peak shapes. The run time is longa (65 min) but provides good conditions for flavonol-glycoside analysis and allows separation of some of the anthocyanin pentosides from known adulterants (e.g., the berries of Vaccinium uliginosum). Based on differences in anthocyanin patterns, bilberry samples from various origins and closely related Vaccinium species can be distinguished. The gradient conditions between 20 and 38 min are not given in the paper, but consist of 10% solvent A - 90% solvent B, before dropping to 89% solvent B at 38 min (Anja Primetta, e-mail communication, August 13, 2015). The column temperature is not indicated. The method has been validated but parameters for system suitability are lacking.
|
|
Chandra24
|
This is
a validated method that can be used to distinguish bilberry from other
anthocyanin-containing ingredients like tart cherry, European elder, and
black chokeberry. The run time is short, but the chromatogram likely contains
a number of unresolved peaks, e.g., delphinidin-3-O-arabinoside and peonidin-3-O-glucoside.
There are no peaks eluting after 16 min, so the run timea can be
shortened. The sample preparation is quick and easy. The use of MS in
addition to the UV detection provides additional information on peak
identity. System suitability parameters are not available.
|
|
Kalt53
|
This
fingerprint method has shown the ability to distinguish bilberry fruit
extracts from extracts of closely related North American Vaccinium species (V. angustifolium,
V. corymbosum, and V. myrtilloides). Despite the long run
time, not all of the peaks are well separated. The column temperature is not
specified. The high injection volume (50 µL) of the sample solution carries
the risk of peak broadening (although the peak shape looks acceptable) and
precipitation of certain components at the injection step. The method has not been validated and
parameters for system suitability are lacking.
|
|
Može, anthocyanins65
|
This
method has a 40-min run timea and has been at least partly
validated (the extent of the validation is not detailed). The method is able
to distinguish bilberry from highbush blueberries (V. corymbosum), but it is not clear if admixtures of the known
adulterants charcoal or amaranth dye would be picked up using the MS
detector. Due to the lack of images, the separation and peak shape cannot be
assessed. The use of an MS as a detection device requires more expensive
instrumentation. Parameters for system suitability are not indicated.
|
|
Može, flavonols, phenolic
acids, and stilbenes65
|
This
method has been at least partly validated (the extent of the validation is
not detailed). The run timea is 60 min but the method can
distinguish bilberry from highbush blueberries (V. corymbosum), although the amounts of most flavonols, phenolic
acids, and stilbenes is rather low in the samples analyzed. It is not clear
how well charcoal or amaranth dye ionizes in negative ion mode and if
admixtures of such adulterants could be detected. Due to the lack of images,
the separation and peak shape cannot be assessed. The use of an MS as a
detection device requires more expensive instrumentation. Parameters for
system suitability are not indicated.
|
|
Penman77
|
The conditions provide a
good separation of bilberry anthocyanins and can be used as a fingerprinting
method for anthocyanin-containing materials. Sample preparation is quick and
easy. The run timea
(42 min) could be shortened, since no peak is eluting after 26 min. The
column temperature is not specified. The method has not been validated and
parameters for system suitability are lacking.
|
|
Buchert78
|
The conditions provide an
acceptable separation of bilberry anthocyanins and can be used as a
fingerprinting method for anthocyanin-containing materials. The sample
preparation using an enzymatic digestion is not applicable to routine QC. The run timea is rather long (70
min). The method has not been validated and parameters for system suitability
are lacking.
|
|
Burdulis79
|
The conditions provide a good separation of
bilberry anthocyanins and can be used as a fingerprinting method for
anthocyanin-containing materials. The sample preparation described applies to
fresh fruit only. The run timea (45 min) could be shortened, since no peak
is eluting after 30 min. The authors specify two different columns for the
separation of anthocyanins, so the actual stationary phase is unclear. The
method has not been validated and parameters for system suitability are
lacking.
|
|
Díaz-García80
|
This
method provides acceptable separation in 25 min.a Some of the
anthocyanins are barely separated (e.g., cyanidin-3-O-glucoside and petunidin-3-O-galactoside).
The method can be used to analyze anthocyanins, flavonols, hydroxycinnamic acids,
hydroxybenzoic acids, flavan-3-ols, and stilbenes in one run. Bilberry fruit
can be distinguished from other anthocyanin-containing fruits (e.g.,
strawberry, sour cherry, cranberry, black grape). A UHPLC system is
prerequisite. The method has not been
validated and parameters for system suitability are lacking.
|
|
Fanali, HPLC-UV81
|
This is
a validated method, although the validation was done using blueberry juice
(no scientific name was given, but the composition of the juice was very
similar to bilberry). The run time is 56 min,a but could be
shortened, since the last peak elutes just before 40 min. Some peaks are
barely separated and the peak shapes of some of the later eluting peaks
(e.g., peonidin-3-O-glactoside) are
less than perfect. Various berry juices can be distinguished based on
anthocyanin profile. System suitability parameters are not indicated.
|
|
Fanali, Nano-LC-ESI-IT-MS81
|
This
method has been validated, although using blueberry juice (see above). The
run timea is fairly short and many peaks are overlapping, but the
use of an MS allows separating the co-eluting anthocyanins based on the
different molecular weights. Various berries can be distinguished based on
anthocyanin profile. The nano-column is not commercially available and has to
be hand-made. The validation data are inferior compared to the HPLC-UV
analysis developed by the same authors. Savings in time and solvents are
offset by the increased costs for the equipment and time used to fabricate
the nano-column. System suitability parameters are not indicated.
|
|
Gardana82
|
This
UHPLC-UV/MS method provides good separation, with only petunidin-3-O-glucoside and malvidin-3-O-galactoside overlapping, although
the run timea of 53 min is on the longer side for a UHPLC method.
The stability of anthocyanins in MeOH-H2O (1:9), which is used for
sample preparation of extracts, needs be established. The sample injection
volume of 50 µL is high and carries the risk of peak broadening (although the
peak shape looks acceptable) and precipitation of certain components at a
flow rate of 0.5 mL/min. The method is able to detect adulteration with black
mulberry, chokeberry, and blackberry. The use of an MS as a detection device
requires more expensive instrumentation, and the
method has not been validated and parameters for system suitability are
lacking.
|
|
Ichiyanagi83
|
The method
provides a good separation with a run timea of 43 min. Due
to the isocratic conditions, the later eluting peaks are very broad, which
may affect quantitative data. The sample preparation is very quick and easy.
There are no data on other anthocyanin-containing materials, including known
bilberry adulterants. The absence of a washout step using isocratic
conditions (20% aqueous methanol) carries the risk of appearance of ghost
peaks in subsequent chromatograms and that the more lipophilic bilberry
components get stuck on the column and thus may shorten its life span. The method has not been validated and parameters for system
suitability are lacking.
|
|
Jovančević84
|
The method
analyzes bilberry anthocyanidins after hydrolysis in 2N HCl at 100°C for 1
hr. The separation is good, but the exact HPLC conditions are unknown (the
method description ends after 22 min, but in the image of the chromatogram,
the peaks for peonidin and malvidin elute after ca. 29 and 31 min,
respectively). There are no data on other anthocyanin-containing materials,
including known bilberry adulterants. The stability of anthocyanins and
anthocyanidins in boiling 2N HCl is not known. The
method has not been validated and parameters for system suitability are
lacking.
|
|
Müller85
|
The
separation in this partially validated fingerprint method is excellent, but
the method is longa (65 min) and has an additional 45 min for
washout and re-equilibration. The internal standard elutes during the washout
period, which is not ideal. The method can distinguish bilberry from highbush
blueberries (V. corymbosum). A
quantification using standard compounds for all 15 bilberry anthocyanins is
not feasible in practice, since this is very expensive and some of the
standards are not commercially available. Parameters for system suitability
are not indicated.
|
|
Nakajima86
|
The
fingerprint method has good separation (the two pairs of overlapping peaks in
UV trace can be separated using extracted ion chromatograms from MS
detection), acceptable peak shapes, and easily distinguishes bilberry, black currant,
chokeberry, and elder berry. The separation timea is 60 min. The
sample preparation is time-consuming, in part due to a purification step
using an Amberlite® XAD-7 resin. The injection volume is missing,
and the stability of the anthocyanin-rich fractions in water needs to be
evaluated. The method has not been
validated and parameters for system suitability are lacking.
|
|
Obón,
anthocyanins87
|
This
reasonably short fingerprint method shows good separation (only peonidin-3-O-arabinoside is missing) and peak
shapes, and easily distinguishes bilberry from seven other
anthocyanin-containing fruits and purple carrots (Daucus carota, Apiaceae). In addition, the method proved its
ability to detect adulteration with seven commercial synthetic and natural
red pigments. The sample preparation is short and simple. The method has not been validated and
parameters for system suitability are lacking.
|
|
Obón,
hydroxycinnamic and hydroxybenzoic acids87
|
This is
a short fingerprint method; however, the optimization for anthocyanins comes
at the price of an insufficient separation for the phenolic acids, in
particular the early eluting hydroxybenzoic acid peaks. Detection using UV at
260 nm or 320 nm is standard in many laboratories, but the more selective
fluorescence detector may have to be added. Based on the various fingerprints,
the authors state that “it is
not possible to use phenolic acid and catechin profiles for the
fingerprinting of a fruit or vegetable juice.”
The method has not been validated and
parameters for system suitability are lacking.
|
|
Yamamoto, HPLC88
|
The
fingerprint method is almost identical to the official methods described by
the USP20 and PhEur13. It has good separation (only one
pair of overlapping peaks in UV trace) and acceptable peak shapes. No
comparison between bilberry and other anthocyanin-containing extracts is
given, but the method has proven its ability to detect adulteration (with
black currant, in this case). The sample preparation is quick and easy. The method has not been validated and
parameters for system suitability are lacking; however, since the only
difference with the USP is the fact that this method starts with a 91%
aqueous phase (rather than 93% as in the USP), the argument can be made that
a full validation is not necessary, and the system suitability parameters can
be adopted from the official method.
|
|
Yamamoto, UHPLC88
|
The
separation of this fingerprint method is comparable to the HPLC method
published by the same authors described above,88 but with the
added advantage of a short run timea of 20 min. The test samples
and sample preparation steps are the same as for the HPLC method. For
laboratories equipped with a UHPLC system, this is a good method for
authentication and to detect adulteration. However, the method has not been validated and parameters
for system suitability are lacking.
|
|
Zhang, fingerprint89
|
This fingerprinting method has a short run timea of 35 min,
although it could be even shorter since the last anthocyanin of interest
elutes before 15 min, and the last peak before 25 min. The short
chromatography time comes at the expense of the separation, since a number of
bilberry constituents are co-eluting. The sample preparation is quick and
easy. The ability to detect adulteration has not been evaluated, but based on
other fingerprinting methods, it should be adequate for the purpose. Once
again, the method has not been validated and
parameters for system suitability are lacking.
|
|
Zhang, hydrolysis89
|
This method has been developed for quantitative analysis
of anthocyanins in bilberry extract. The anthocyanidins are separated under
isocratic conditions after hydrolysis. The separation is good, although the
isocratic conditions lead to an obvious broadening of the later-eluting
peaks. The sample preparation is more time-consuming due to the hydrolysis
step. The fact that only anthocyanidins are measured impacts the ability of
this method to distinguish bilberry extracts from other
anthocyanidin-containing extracts, and as such, it is not adequate as a means
to detect adulteration. The method has not been
validated and parameters for system suitability are lacking.
|
aThe
run times do not include the time used to return to initial conditions and
equilibrate, since this information is not always provided in the publications.
Note:
The term “validated” is used when a method has been validated for quantitative
analysis, but not in terms of qualitative identification according to LaBudde
and Harnly.94
Table
5. Comparison of different published HPLC methods for V. myrtillus. Sample preparation steps and times are indicated only
for dry bilberry extracts, not for fresh fruit or fruit juice.
|
Reference
|
Number of samplesa
|
Origin of samples
|
Sample preparation: handlingb /
duration [min]c
|
Column Type
|
Run time [min]d
|
Detection wavelength (UV) or ion mode (MS)
|
|
EP 8.4,13 USP 38-NF 33,20 Govindara-ghavan,32 Cassinese76
|
432
|
USP
powdered bilberry extract RS (1), commercial bilberry extracts (3)32
|
4/4520
5/5013,32,76
|
C18
|
50
|
UV: 535
|
|
Lätti22
|
179
|
Harvested
by authors
|
n/a
|
C18
|
65
|
UV: 520
|
|
Chandra24
|
1
|
Commercial
raw material
|
4/30
|
C18
|
26
|
UV: 520
MS
(positive)
|
|
Kalt53
|
1
|
Commercial
fresh fruit
|
n/a
|
C18
|
65
|
UV: 280,
520
|
|
Može, anthocyanins65
|
7
|
Harvested by authors
|
n/a
|
C18
|
40
|
MS
(positive)
|
|
Može, flavonols, phenolic
acids, and stilbenes65
|
7
|
Harvested
by authors
|
n/a
|
C18
|
60
|
MS
(negative)
|
|
Penman77
|
2
|
Commercial
raw material
|
4/45
|
C18
|
42
|
UV: 540
|
|
Buchert78
|
1
|
Commercial
fresh fruit
|
n/a
|
C18
|
70
|
UV: 520
|
|
Burdulis79
|
11
|
Harvested
by authors
|
n/a
|
C18
|
45
|
UV: 520
|
|
Díaz-García80
|
1
|
Commercial
fruit juice
|
n/a
|
C18
|
38.8
|
UV: 520
|
|
Fanali, HPLC-UV81
|
-
|
Commercial
fruit juice
|
n/a
|
C18
|
56
|
UV: 518
|
|
Fanali, Nano-LC-ESI-IT-MS81
|
-
|
Commercial
fruit juice
|
n/a
|
C18
|
33
|
MS
(positive)
|
|
Gardana82
|
45
|
Commercial
frozen fruit (19), extracts (14), capsules (6), and juices (6)
|
17/55
(extracts)
|
C18
|
53
|
UV: 520
MS
(positive)
|
|
Ichiyanagi83
|
1
|
Commercial
product
|
3/15
|
C18
|
43
|
UV: 520
|
|
Jovančević84
|
11
|
Harvested
by authors
|
n/a
|
C18
|
unclear
|
UV: 520
|
|
Müller85
|
13
|
Commercial
fruit and fruit juice
|
n/a
|
C18
|
65
|
UV: 520
|
|
Nakajima86
|
1
|
Commercial
frozen fruit
|
n/a
|
C18
|
60
|
UV:
500-550 MS (positive)
|
|
Obón,
anthocyanins87
|
1
|
Commercial
fruit juice
|
n/a
|
C18
|
38
|
UV: 520
|
|
Obón, hydroxycinnamic and hydroxylbenzoic
acids87
|
1
|
Commercial
fruit juice
|
n/a
|
C18
|
38
|
UV: 260,
320; Fluorescence
|
|
Yamamoto, HPLC88
|
14
|
Commercial
bilberry extracts (11) and bilberry combination products (3)
|
4/45
|
C18
|
50
|
UV: 535
|
|
Yamamoto, UHPLC88
|
14
|
Commercial
bilberry extracts (11) and bilberry combination products (3)
|
4/45
|
C18
|
20
|
UV: 535
|
|
Zhang, fingerprint89
|
2
|
Commercial
bilberry extracts
|
4/35
|
C18
|
35
|
UV: 525
|
|
Zhang, hydrolysis89
|
2
|
Commercial
bilberry extracts
|
6/125
|
C18
|
30
|
UV: 530
|
aNumber of bilberry samples
analyzed
bNumber of sample preparation
steps involved (see Appendix 1)
cEstimated based on
description provided in the reference (see Appendix 1)
dNot including the time used
to return to initial conditions and equilibrate
n/a: not applicable
|