NOVEMBER 2018  Click here to view PDF Click here to view PDF
Cranberry Products
Laboratory Guidance Document
By John H.
Cardellina II, PhDa and Stefan Gafner, PhDb
a ReevesGroup,
Virginia Beach, VA 23451
b
American Botanical Council, PO Box 144345, Austin,
TX 78714
Correspondence: email
Citation (JAMA style): Cardellina II
JH, Gafner S. Cranberry products laboratory guidance document. Austin, TX:
ABC-AHP-NCNPR Botanical Adulterants Prevention Program. 2018.
Keywords:
Adulteration, Arachis hypogaea, cranberry, cranberry
fruit extract, cranberry juice, grape seed extract, peanut skin extract, pine
bark extract, Pinus massoniana,
proanthocyanidin, procyanidin, Vaccinium macrocarpon,
Vitis vinifera CONTENTS 1. Purpose 2. Scope 3. Common and Scientific Names 3.2 Other common names 3.3 Accepted Latin binomial 3.4 Synonyms 3.5 Botanical family 4. Botanical Description 5. Identification and Distinction of Fruit Using Macroanatomical Characteristics 6. Identification and Distinction of Fruit Using Microanatomical Characteristics 7. Genetic Identification and Distinction 8. Cranberry Products Description Figure 1. Flow diagram of cranberry fruit processing, illustrating various ingredients and products of cranberry 9. Chemical Identification and Distinction 9.1 Chemistry of V. macrocarpon fruit Figure 2. Representatives of the main classes of secondary metabolites in cranberry 9.2 Chemistry of potential cranberry adulterants Table 1: Proanthocyanidin characteristics of low-cost, non-cranberry botanical materials containing condensed tannins 9.3 Laboratory methods Table 2. Comparison of different analytical approaches to determine adulterants in cranberry products Figure 3: HPTLC analysis of cranberry, related and adulterating species Figure 4: HPTLC analysis of cranberry, related and adulterating species Figure 5: HPTLC analysis of cranberry, related and adulterating species 10. Conclusions 11. References
1. Purpose
Cranberry remains one of
the most popular of the ‘healthy’ fruits, with an array of extract products
appearing in the botanical dietary supplement markets and a large number of
juice products in the beverage industry. There is considerable evidence that
both, but especially the extract-based product categories have been subjected
to adulteration.1 This Laboratory Guidance Document is intended to review
the analytical technologies used to determine whether cranberry extract
products are authentic and, if not, to identify the adulterants involved. This
document should be viewed in conjunction with the corresponding Botanical Adulterants Bulletin on
Cranberry published
by the ABC-AHP-NCNPR Botanical Adulterants Prevention Program.1
2. Scope
The continued demand for
cranberry based supplements and beverages in the marketplace and the rising
costs of cranberry raw material have seemingly served as an incentive for
economically motivated adulteration with synthetic colorants and/or anthocyanin-
or proanthocyanidin (PAC)- rich extracts or PAC-rich materials (e.g., powders) from
other, less expensive botanical sources. While admixture or substitution with
synthetic colorants or anthocyanin-containing extracts can be detected rather
readily, the inclusion of PACs from, for example, grape seed, peanut skin, or
pine species in products purported to be cranberry extract is more difficult to
detect and may require more advanced instrumentation, and/or a combination of
analytical methods.
The evaluation of a
specific analytical method or methods in this Laboratory Guidance Document for
testing cranberry materials does not reduce or remove the responsibility of
laboratory personnel to demonstrate adequate method performance in their own
laboratory using accepted protocols outlined in various domestic (in the United
States) or international legal and/or regulatory documents. Such documents
include, for example, the 21 CFR Part 111 (Dietary Supplement GMPs, in the US
Code of Federal Regulations) and Part 117 (FSMA Final Rulemaking for Current
Good Manufacturing Practice and Hazard Analysis and Risk-Based Preventive
Controls for Human Food, in the US Code of Federal Regulations), and by AOAC
International, International Standards Organization (ISO), World Health
Organization (WHO), and the International Council on Harmonisation (ICH).
3. Common and Scientific Names
3.1
Common name: cranberry
3.2
Other common names
English:
American
cranberry, large cranberry, North American cranberry2-5
Chinese:
da guo yue jie (大果越桔)6
French: canneberge, canneberge d’Amérique,
canneberge à gros fruits, atoca, atoka, ronce d’Amérique2,3
German: Kranbeere,
grosse Moosbeere2-4
Italian: ossicocco
americano, mirtillo rosso canadese, mortelle di palude, cranberry7
Spanish: arándano,
arándano americano, arándano trepador, arándano rojo2-4
Wampanoag:
ibimi, sasumuneash8
3.3
Accepted Latin binomial:
Vaccinium macrocarpon Aiton
Note: Cranberry products
on the dietary supplement, food and beverage markets are predominantly made
from V. macrocarpon. However, the American
Herbal Products Association’s (AHPA) Herbs
of Commerce, 2nd edition, which
provides guidance on dietary supplement labeling in the United States, also
permits products derived from V. oxycoccos
to be labeled as cranberry.9
3.4
Synonyms: Oxycoca macrocarpa (Aiton) Raf., Oxycoccus macrocarpus (Aiton) Pers., Oxycoccus palustris var. macrocarpos (Aiton) Pers., Schollera macrocarpa (Aiton) Steud., Schollera macrocarpos (Aiton) Britton
3.5
Botanical family: Ericaceae
4.
Botanical Description
Vaccinium macrocarpon, which is indigenous to North America, is a fruit bearing, trailing or
ascending rhizomatous evergreen shrub that grows 5-20 cm in height. Cranberry
plants in the wild are generally associated with bogs, swamps and other low-lying
wetland areas; the species has adapted to low nutrient, generally sandy soils.8
The fruit (berry) is the only component of current interest or importance in
trade, although there are some references to Native American use of the stem
and leaves for medicinal purposes.8
5.
Identification and Distinction of Fruit Using Macroanatomical Characteristics
Fresh berries
are globose to ellipsoidal; 9 to 20 mm in diameter; red, crimson, burgundy to
almost black; glabrous, with a smooth lustrous surface. A more detailed physical
description is available in the American Herbal Pharmacopoeia (AHP) monograph
on cranberry.8 The morphological features may allow one to
distinguish the fruit of V. macrocarpon
from fruits of V. oxycoccos and V. vitis-idaea (the latter two species have
berries of smaller size [V. oxycoccos:
9-14 mm; V. vitis-idaea: 6-12 mm; and
V. macrocarpon: 9-16 mm] and globose
compared to the slightly elongated V.
macrocarpon berry),10 but for cranberry powders and extracts,
where adulteration issues are most prominent, macroscopic identification is not
feasible.
6.
Identification and Distinction of Fruit Using Microanatomical Characteristics
The exocarp is
comprised of anthocyanin-colored polygonal cells covered by a thick cuticle.
Groups of cells are separated by fairly thick, colorless walls, while the walls
within the respective groups are rather thin. The mesocarp consists of large,
spherical, thin-walled cells in which small bundles of spirally thickened
vessels are embedded. As noted above in Section 5, a more detailed description,
including figures displaying key anatomical features, is available in the AHP
monograph on cranberry, and from other sources.8,11,12 Microscopic distinction
of V. macrocarpon, V. oxycoccos, and V. vitis-idaea may not be feasible, although no papers
investigating the topic could be retrieved. Botanical microscopy is not capable
of detecting adulteration with extracts from other plant sources.
7.
Genetic Identification and Distinction
While there have been a
number of genetic studies of V.
macrocarpon using simple sequence repeats (SSR) in recent years, they have
all been focused on identifying genetic characteristics related to fruit
quality and breeding programs.13-17 Researchers in Lithuania and
Poland used both SSR and RAPD (random amplified polymorphic DNA) to compare two
wild populations of V. oxycoccos
growing in different nature preserves in Lithuania.18 The authors
reported 71% variation between the two populations, based on RAPD analyses,
compared to 97% variation in the SSR comparison. Unfortunately, no genetic
comparison of different Vaccinium
species were conducted in these or other such studies in the literature.
A preliminary report on an
assay analyzing DNA by PCR amplification of the MatK gene was recently presented by Herbst and co-workers. The
authors reported successfully discriminating V. macrocarpon DNA from DNA of grape (Vitis vinifera, Vitaceae), apple (Malus domestica, Rosaceae) and pear (Pyrus spp., Rosaceae); unfortunately, the primers developed thus
far were unable to distinguish cranberry from blueberry (V. corymbosum).19 Further work by this group may lead to
a genetic means of distinguishing various Vaccinium
spp. in commerce.
8.
Cranberry Products Description
Cranberry products may be
comprised of powdered whole berries, juice, juice concentrate, juice powder,
powdered pomace (residue after juice pressing), dried pomace extracts, and
processed juice fractions. A flow diagram of the various processing steps for
cranberries is provided in Figure 1.
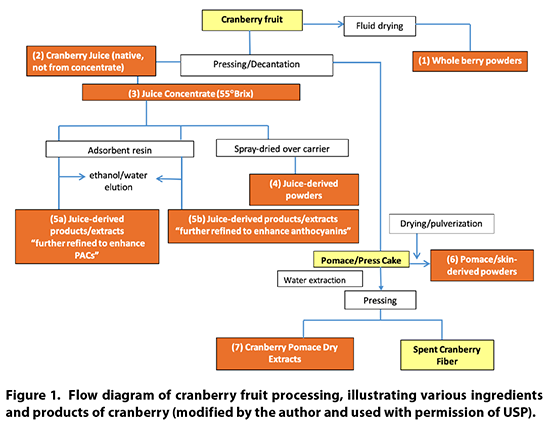
9.
Chemical Identification and Distinction9.1
Chemistry of V. macrocarpon fruit A good summary of the
chemistry of cranberry is provided in the AHP monograph.8 The
chemistry is dominated by phenols and polyphenolics, notably anthocyanins and
procyanidins*. The procyanidins are oligomers† and polymers of
catechins, each connected to another by either two bonds (A type) or one bond
(B type); the A type procyanidins have been identified as the putative
bacterial anti-adhesion compounds in cranberry, while the B type PACs have been
shown to be inactive as anti-adhesion agents.20 There are four known
catechin (flavan-3-ol) building blocks in cranberry, and oligomers of three or
more catechin units are considered the pharmacologically active procyanidins;
the challenge of rigorously identifying the complete structure and absolute
configuration of a PAC is directly proportional to the degree of polymerization
(DP), i.e., the number of catechin units present, as the number of possible
isomers increases with increasing DP. The anthocyanins provide cranberry with
its red color; there are six major anthocyanins in cranberry, which are
glycosides of two anthocyanidin aglycones (cyanidin and peonidin) and three
sugars (galactose, arabinose and glucose, listed here in order of abundance in
cranberry anthocyanins). Also abundant in cranberry
are flavonols. While more than 20 flavonol glycosides have been identified in
cranberry, the primary flavonol glycosides are galactosides, arabinosides, and rhamnosides
of quercetin, myricetin, and kaempferol. Certain processing operations can
release the flavonol aglycones and free sugars in the final product or
ingredient, e.g., via hydrolysis. Another important group of compounds, from
the standpoint of identification and adulteration, is the organic acids, mainly
quinic, citric, and malic acids. Particularly noteworthy is the high relative
level of quinic acid in cranberries; analysts can make use of the ratios of
quinic to the other acids to glean insight into potential adulteration of
cranberry juice or dietary ingredients derived from juice. Triterpenes are also
found in cranberry; ursolic acid is the most abundant of these, although a
number of structurally related pentacyclic triterpenes are also present in the
fruit and leaves. Figure 2 illustrates the most important chemical classes
found in cranberry.
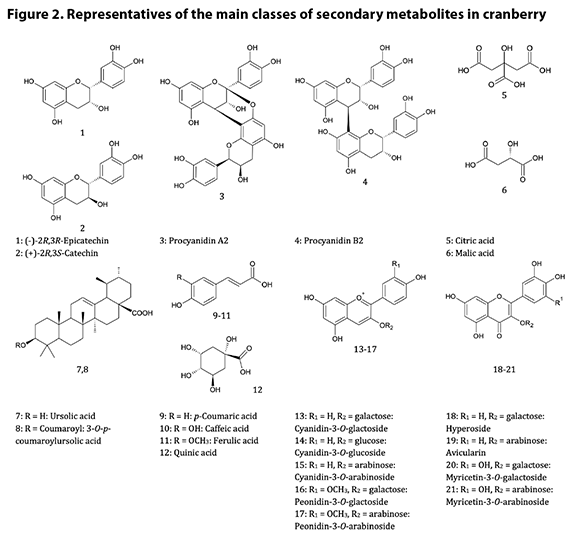 9.2
Chemistry of potential cranberry adulterants
While anthocyanins from
grape (Vitis vinifera, Vitaceae) seed
and skin extracts were detected in cranberry juice over 30 years ago, more
accurate labeling of juice products to acknowledge admixture of other fruit
juices has reduced the problem of adulteration of juices. However, there
remains the possibility that other fruit juices can be masqueraded as cranberry
juice by the addition of anthocyanins and, perhaps, quinic acid, from exogenous
sources.
Adulteration of dried
cranberry concentrates and powdered extracts is considered more common, driven
by increasing consumer demand for and rising prices of cranberries, and abetted
by a dearth of suitable, broadly applicable analytical methods and lack of
reference compounds. Potential cranberry adulterants will likely mimic either
the anthocyanin fraction or the PAC fraction, the focus of most marketing
efforts. It thus follows that reported adulterants include grape seed and skin
extracts, red peanut (Arachis hypogaea,
Fabaceae) skin extracts, plum (Prunus
domestica, Rosaceae) extracts, and,
to a lesser extent, extracts of maritime pine (Pinus pinaster, Pinaceae) and Masson pine (P. massoniana) bark, black bean (Phaseolus vulgaris, Fabaceae) skins, black rice (Oryza sativa, Poaceae), mulberries (Morus spp., Moraceae), and other parts
of cranberry plants.8
Vitis vinifera: Grape seed extract (GSE) is almost
exclusively supplied to dietary supplement manufacturers in the form of a dry
extract. The extract contains polyphenolic compound concentrations in a range
of 50-90% of the extract. The main phenolic compounds are flavan-3-ol monomers
and polymers and their gallic acid esters. Grape seeds contain predominantly
B-type PACs, which are flavan-3-ol polymers where the units are linked by a
single bond. Appeldoorn et al.21 isolated procyanidin B1, B2, B3,
and B4 from a commercial GSE, accounting for 3.2%, 7.1%, 1.5%, and 1.2% of the
extract. Similar results were reported by Weseler and Bast,22 with
concentrations of 7.7%, 8.3%, 2.8%, and 1.6% of procyanidins B1, B2, B3, and
B4, respectively. The presence of B-type dimers, trimers, tetramers and
polymers of up to the size of a dodecamer trigallate was described by Weber et
al.,23 who analyzed four commercial GSEs by HPLC-APCI/MS, and
MALDI-TOF/MS and found that the molecular weight distribution varied
substantially depending on the product. Average degrees of polymerization (DP)
for commercial GSE were reportedly between 3-11,24,25 although the
DP may deviate substantially from these values, depending on processing.
Arachis
hypogaea: Peanut skin extracts contain both A-type and B-type
PACs.26,27 Appledoorn isolated
a number of PACs from peanut skin, with A-type dimers procyanidin A1 and A2 as
most abundant (6.9% and 2.1%, respectively). Procyanidin B7 was present at
0.2%.21 Dudek et al.28 confirmed the presence of
procyanidins A1 and A2, and isolated four trimers and two tetramers, named
peanut procyanidins A-F. Besides procyanidin A1, peanut procyanidin E was the
most abundant in a 70% aqueous acetone extract of the skins. Other phenolic
compounds in peanut skin include flavonols (quercetin, kaempferol,
isorhamnetin, and their glycosides), the isoflavone genistein, and their
glycosides), the isoflavone genistein, the flavanone hesperetin, anthocyanins
(cyanidin, cyanidin-3-O-glucoside,
cyanidin-3-O-sophoroside, peonidin-3-O-galactoside, and petunidin-3-O-galactoside), and the stilbene
resveratrol.29
Pinus spp.: Weber et al.23 also (see Vitis vinifera, above) investigated
the PAC type and size in extracts from P.
pinaster and P. massoniana. From
an economic perspective, Masson pine extracts are 5-10 fold less expensive than
Maritime pine bark extracts, making Masson pine more attractive as an economic
adulterant (Yannick Piriou [les Dérivés Résiniques et Terpéniques] email to Maria J. Monagas [USP], May 3, 2018). Contrary to
Maritime pine, Masson pine contains some galloylated PACs.23 The
monomer units consist mainly of catechin and epicatechin, although small
amounts of epigallocatechin and gallocatechin have also been reported.30,31
Typically, pine bark extracts contain only B-type PACs. The average degree of
polymerization of a hot water extract of P.
pinaster is between 6 and 7.30,32 Similar results were reported
for Scots pine (Pinus sylvestris) by
Bianchi et al.33 The PAC fraction of a hot water extract consisted
of exclusively of B-type procyanidins with average degree of polymerization of
6.7. A comparison of HPLC-UV fingerprints between grape seed and Masson pine
extract did not show a substantial difference, except that the Masson pine
extract had a larger concentration of more highly polymerized PACs and
exhibited the peak of an A-type dimer.34 Table 1 lists the key
characteristics of the PAC constituents of the adulterant botanicals described
above.
Table
1: Proanthocyanidin characteristics of low-cost, non-cranberry botanical materials
containing condensed tannins
|
Ingredient
|
Monomer(s)
|
Galloylation
|
PAC-type
|
Average
degree of polymerizationa,b
|
|
grape seed
|
catechin, epicatechin
|
Yes
|
B-type
|
2-1224,25,35
|
|
almond skin
|
afzelechin, catechin,
gallocatechin, epiafzelechin, epicatechin, epigallocatechin
|
No
|
A-type, B-type
|
no data
|
|
apple
|
catechin, epicatechin
|
No
|
mainly B-type
|
3-1036,37
|
|
green tea
|
catechin, epicatechin,
epiafzelechin, epigallocatechin, gallocatechin
|
Yes
|
B-type
|
1-1.138
|
|
maritime pine
|
catechin, epicatechin,
epigallocatechin, gallocatechin
|
No
|
B-type
|
3-732
|
|
Masson pine
|
catechin, epicatechin,
epigallocatechin, gallocatechin
|
Yes
|
mainly B-type
|
no data
|
|
peanut skin
|
catechin, epicatechin
|
No
|
A-type, B-type
|
1-939
|
a Measured by thiolysis
b Degree of polymerization determined depends on the
processing method; for grape seed, degrees of polymerization between 1 and 37
have been reported on isolated fractions40-42
9.3
Laboratory methods
There are various reports
in the literature on analytical methods to identify cranberry, assess its
quality, and/or disclose evidence of adulteration. Analytical methods for the
analysis of main cranberry polyphenols (monomeric flavan-3-ols and flavan-3-ol
glycosides, anthocyanins, and PACs), sugars, and organic acids have been
developed. Not all the reported methods are suitable for all these purposes or
all forms of cranberry ingredients in the marketplace. The selection of
anti-adulteration analytical methods is largely dependent on the composition of
each ingredient or finished product, which at the same time defines testing
requirements for quality assurance purposes. Cranberry juice or juice
concentrate could be assessed following official juice standards — European
Juice Association (AIJN) Code of Practice Reference Guidelines for Cranberry
Juice;43 USDA Commodity Specification Bottled Juices – Cranberry
Juice Concentrate 3+1 (commercial name: Cranberry Juice Cocktail) and Cranberry
Juice Concentrate 55-gallon drum (commercial name: Cranberry Juice Concentrate
50 Brix);44 USP-NF Cranberry Liquid Preparation; Codex General
Standard for Fruit Juices and Nectars (CODEX STAN 247-2005)45 — by
considering the ratio of organic acids and sugars, as well as the anthocyanin
profile. Some of these tests could be applied to cranberry spray-dried juice powders
and whole berry powders also, as the original fruit identity is still present
in this type of ingredient. However, when juices are further processed and
purified into cranberry extracts (for example, by industrial resin adsorption)
the original juice identity (chemical profile) is altered and other tests are
required to properly characterize the ingredient. This is also the case for
ingredients derived from aqueous extraction from the pomace remaining after
juice extraction (cranberry pomace extracts) and the skin-derived powders. In
these latter cases, the proper characterization of the PAC fraction becomes critical
to the detection of adulteration.
Table 2 provides a
selection of representative analytical methods used to analyze commercial cranberry
products that seem most adaptable for investigations of adulteration and
considers the key advantages and disadvantages of each.
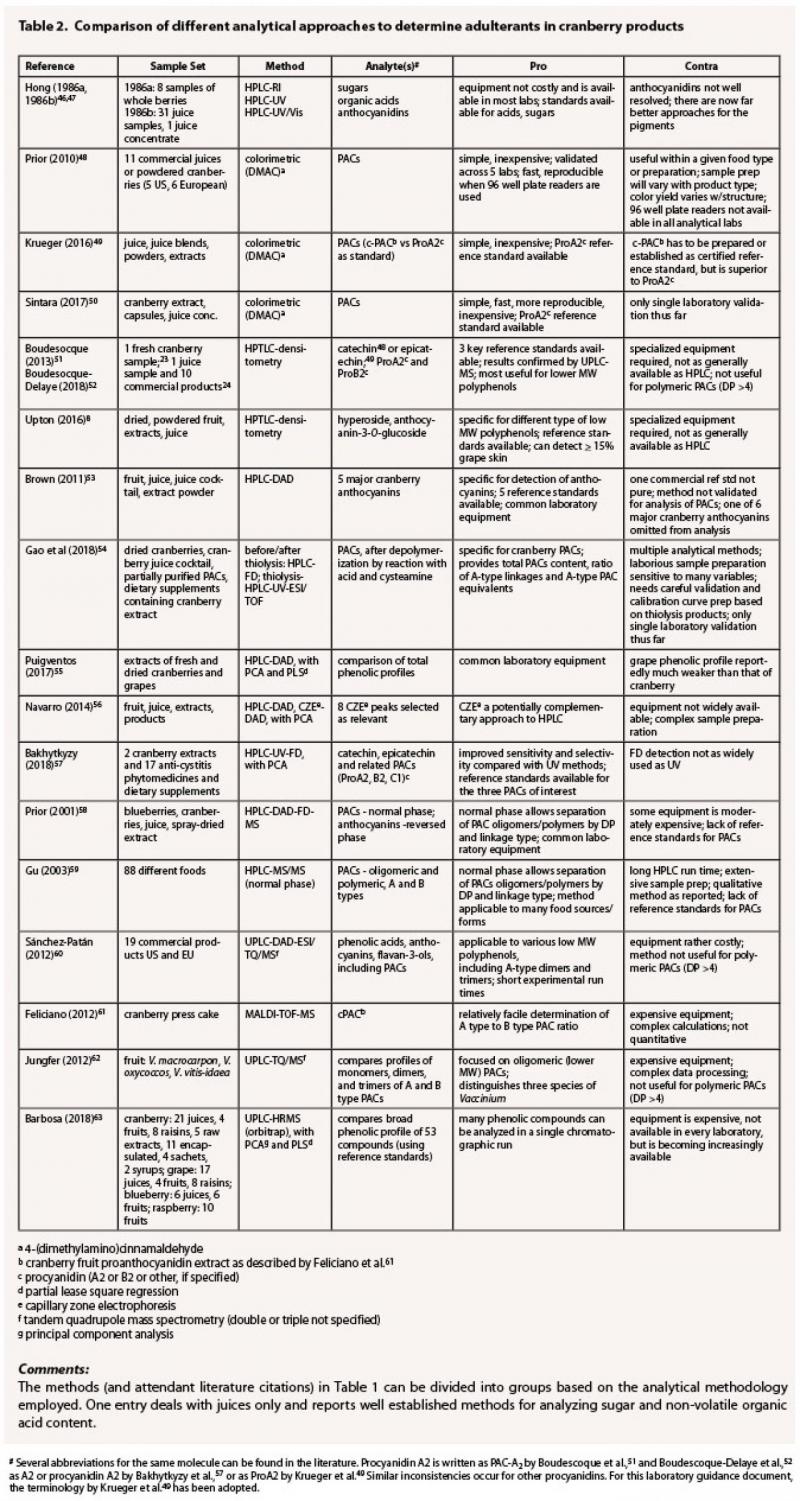
The challenge with juices
is determining what adulterant juices or additives (e.g., sugars, organic acids,
pigments) are present. A variety of options is available to
researchers/analytical groups, but the most useful of these appear to be
analysis of sugar content (notably glucose and fructose) and organic acid
content (quinic, malic, and citric). Hong and Wrolstad used HPLC-RI and HPLC-UV
to identify anomalies in the sugar and acid constitution of purported cranberry
juices over three decades ago, coupled with HPLC-UV analyses of the anthocyanidin
profiles. They identified 20 of 31 juice samples that they analyzed as
adulterated.46,47 Reviewing these two articles is informative, as
the sugar and organic acid methodologies are still useful today, offering good
resolution, but the separation of the anthocyanidins as presented in the paper seems
quite dated by today’s standards, and yet the researchers could readily
distinguish cranberry juice from those of blackberry and mango. Current HPLC
column technology and the use of MS as the detection mode permit direct
analysis of the anthocyanins in cranberry and other fruits.
It is important to note
that analyzing the anthocyanin profile is an excellent check for adulteration
by color, i.e., adding exogenous colored materials to present an apparent
cranberry color. Brown and Shipley53 developed and validated, via
single laboratory protocol,64 a quantitative HPLC-DAD analysis of
the five major anthocyanins of cranberry as a quality control tool. Reference
standards of those anthocyanins are commercially available, permitting
verification of identity and quantitative measurements of content in fruit,
juice, juice cocktail, and dried extracts. This method is an interesting
complement to the numerous methods to measure the content of the PAC in
cranberry and can be used to assess overall quality and composition of
cranberry products. More recently, 12C/13C
ratios have been increasingly used to identify cases where synthetic or
exogenous sugars have been added to a juice product.65 The rest of the entries
are focused on the polyphenolics (PAC or total phenolic profile) in cranberry;
analytical approaches include colorimetric (DMAC), HPTLC-densitometry, HPLC-UV
and/or FD, HPLC-MS, and MS (MALDI TOF, Orbitrap). The DMAC assay, which
involves the reaction of 4-dimethylamino cinnamaldehyde at a hydrogen-bearing
aromatic carbon with two free phenolic hydroxyl groups positioned ortho- or
ortho-/para- in the flavanol portion of a PAC molecule to form an intensely green-
or blue-colored compound, has been the subject of investigation as a potential
quantitative assay for PAC for about two decades. The three DMAC papers listed
in Table 1 highlight recent developments with this assay. Prior et al.48 validated
a DMAC method across five laboratories, using a sample set (N = 11) consisting
of juices or powdered fruit or extract. They used commercially available procyanidin-A2
as a standard; cranberry powders (dried, ground berries) were extracted in a
protocol that required 1-1.5 hours of effort, while the PAC fraction of juice
was obtained by quick chromatography on C18 cartridges. The DMAC
reaction was conducted and the color read and evaluated in a 96 well plate format.
Krueger et al.49 followed the Prior study with a comparison of the
use of procyanidin-A2 vs c-PAC, a standardized total PAC fraction from
cranberry press cake (pomace) extracts, as a standard for the DMAC assay. Their
investigation revealed more accurate results with the use of c-PAC, but the
preparation of c-PAC was labor intensive, involving a triple extraction and gel
permeation chromatography. The challenge, then, would be to create a
significant, sustained supply of certified reference standard c-PAC, in order
for results from different laboratories to be compared. Very recent work by
Sintara et al.50 reports a single laboratory validation of a DMAC
method using procyanidin-A2 as a reference standard. Improvements over previous
methods include changing extraction solvent to methanol for better
reproducibility, changing solvent for DMAC reagent from hydrochloric acid in
ethanol to sulfuric acid in methanol for higher sensitivity, and using a UV/Vis
spectrophotometer instead of a plate reader for wider availability. The
precision of the DMAC method was improved from 16.5% (RSD for Prior48)
to an RDS of less than 5%. However, the DMAC method, as well as other spectrophotometric
methods, is not appropriate for the detection of adulteration since it is not
specific enough to differentiate among cranberry PACs, and those from potential
adulterants. Two papers illustrate the
evolution of an HPTLC-densitometry approach by Boudesocque-Delaye et al.51,52
In the first, catechin, procyanidin-A2 and procyanidin-B2 served as
reference standards, while epicatechin replaced catechin in the more recent
iteration of the methodology. In the latter paper, the authors report that the
sample preparation protocol, which required several liquid/solid extractions to
isolate fully the polyphenolic fraction, was crucial to obtaining a meaningful
comparison of product quality and pharmacological activity. The
HPTLC-densitometry results, which indicated that only two of the 10 products
tested were high quality cranberry formulations, were confirmed by both UPLC
and DMAC analyses. The recently revised AHP monograph on cranberry fruit by
Upton and Brendler8 provides a richly detailed description of the
sample preparation, execution and review of an HPTLC analysis of various forms
of cranberry, including samples adulterated with 15% grape skin extract by
weight; a series of informative color plates are included. HPTLC is a good
screening technique, allowing the detection of most types of adulteration,
although mixtures/substitutions between cranberry and certain other PAC-rich
extracts can represent a problem. However, some of these materials may be
distinguished from cranberry based on the general fingerprint, or by comparing
the flavan-3-ol monomer, dimer, and trimer pattern (Figures 3-5)
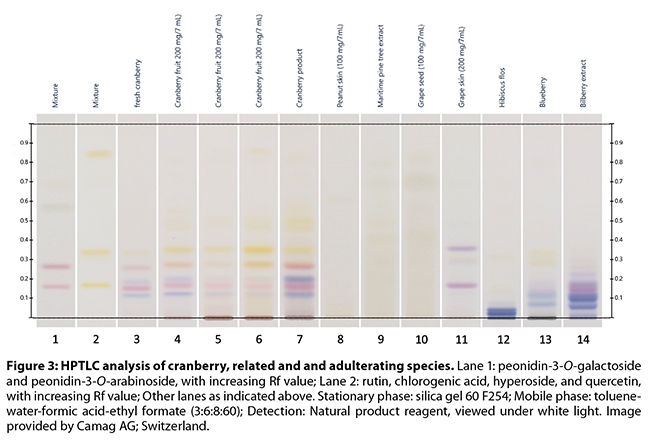
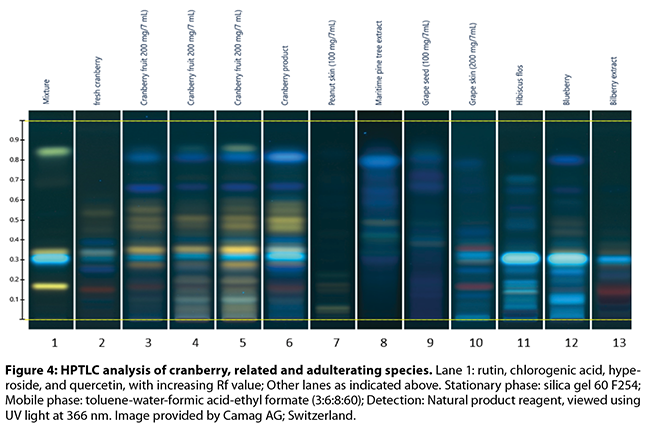
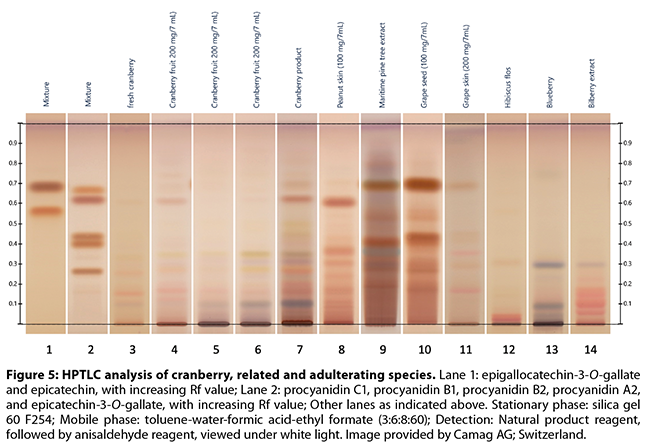
Brown and Shipley’s53
single laboratory validated quantitative HPLC-DAD analysis of the five major
anthocyanins of cranberry as a quality control tool is discussed above in
regard to juice and juice products. This method is an interesting complement to
the numerous methods to measure the content of PACs in cranberry. In a quite
different approach, Puigventos et al.55 used HPLC-DAD, followed by
PCA and PLS data analyses, to compare the entire polyphenolic profiles of
extracts of both fresh and dried cranberries and grapes. The grape polyphenolic
profile was significantly weaker than the cranberry profile at the three
wavelengths evaluated (280, 370 and 520 nm), but was most prominent at 370 nm.
Application of PCA and PLS data mining allowed distinction of test mixtures
containing 50 or 10% grape juice (in cranberry juice), but 2.5, 5, and 7.5%
grape juice ‘adulteration’ could not be differentiated from pure cranberry
juice. Gao et al.54
revived and modified a 45-year-old thiolysis method66,67 and
combined that with HPLC-FD detection to develop a method to quantitate total
procyanidins, average degree of polymerization, ratio of A-type linkages, and
A-type procyanidin equivalents in cranberries, cranberry juice, partially
purified PACs and dietary supplements containing cranberry extracts. While the
sample preparation is sensitive to a number of variables, the method has been
through an AOAC single laboratory validation and offers the distinct advantage
of an ability to focus on the A-type PACS, because the thiolysis reaction is
blocked from cleaving the carbon-carbon bond that distinguishes A-type PACs.
The authors used HPLC-ESI/TOF to verify the composition of the various
thiolysis products. Bakhytkyzy et al.57 established an HPLC method
using fluorescence detection (FD) to separate and identify A, B and C type
PACs; one of the keys to success in this method was the availability of
authentic reference standards for procyanidin-A2, -B2, and -C1, along with
catechin and epicatechin. FD gave better sensitivity and selectivity than UV
detection. The authors found that the two extracts and 17 market products they
analyzed fell into three groups: a) extracts rich in procyanidin-A2; b)
extracts enriched in monomeric species; and c) extracts rich in procyanidin-B2.
These results indicated that a significant number of the analytical samples did
not conform to expectations of a cranberry profile. Prior et al.58
used HPLC-DAD-FD-MS to profile both the PAC (normal phase HPLC) and anthocyanin
(reverse phase HPLC) content of cranberries and blueberries, along with their
juices and extracts. The authors observed the best separation/resolution of the
PACs by normal phase HPLC, while the anthocyanins were readily resolved by the
more commonly used reverse phase columns. The compounds were detected by both
UV (DAD) and FD, while compound identities were confirmed by comparison of
retention time and mass spectral data, when reference compounds were available,
or were proposed by comparison of UV and mass spectral data with literature
reports, when standards were not available. Gu et al.59 used
a similar normal phase HPLC-MS/MS method to analyze different food forms for
oligomeric and polymeric PACs; the complexity of the sample preparation and the
long HPLC run times may limit adaptation of this method to the food industry. Sánchez-Patán
et al.60 applied different reverse phase UPLC-DAD methods for the
separation and analysis of phenolic acids/flavan-3-ols (including PACs) and
anthocyanins by tandem quadrupole MS; this approach allowed the researchers to
demonstrate that only four of 19 commercial extract products examined delivered
the requisite daily dose of 36 mg of PACs. The method has a relatively
straightforward sample preparation and short run time, but the latter is offset
by having to run two separate UPLC analyses to account for all the analytes of
interest. Feliciano et al.61
used MALDI-TOF MS to determine the ratios of PAC-A to PAC-B in cranberry press
cake and juice; however, the method is not quantitative and requires extensive
calculations. Even though no commercial products were analyzed, the contribution
from Jungfer et al.62 is of considerable potential value, because it
compares the profiles of the monomers, dimers, and trimers of A and B type PACs
in three species of Vaccinium: V. macrocarpon, V. oxycoccos, V. vitis-idaea.
The researchers used UPLC and triple quadrupole MS to establish the unique
profiles in each species and the variation observed in samples of different
origin. This method would seem to have great usefulness in species verification
at the raw material acquisition stage. However, analysts must remember that the
total amount of PACs as monomers, dimers and trimers represents only a fraction
of the total PACs in any cranberry product. In a very recent paper, Barbosa et
al.63 utilized UPLC-HRMS (Orbitrap system) to create and compare
phenolic profiles, using 53 reference standard compounds, of cranberry, grape,
raspberry and blueberry fruit, dried fruit, juice, extracts and finished
products. As might be expected, grape was easily differentiated from cranberry,
with raspberry showing similar, but not as significant differences. Blueberry
and cranberry were closely related in the PCA analyses illustrated in the
manuscript. Cranberry extracts and encapsulated products showed significant
differences from fruit, juice and dried fruit; this is not surprising, given
the alteration of chemical profile brought on by extraction and other
processing steps. PLS regression analyses were efficient in identifying the
level (%) adulteration in mixtures of grape and cranberry juices. This method
also has considerable potential for the detection and identification of
adulteration of cranberry products. HPLC-UV/MS is appropriate for the detection
of most types of adulteration if it is based on a fingerprint of flavan-3-ol
monomers, dimers, smaller oligomers, and other relevant compounds. One
advantage having a UV/Vis detector as part of a hyphenated system is that it
can measure anthocyanins at the same time. Based on the paper by Ye et al.,68
distinction between cranberry and peanut skin extracts may be challenging, even
using a MALDI-TOF MS fingerprint. The latter is nevertheless ideal for
distinguishing PAC-rich materials from various sources, but may not be optimal
for materials with little to no PACs, such as green tea. In addition,
adulteration of cranberry extracts with anthocyanin-rich materials, or with
food colorants may go undetected using MALDI-TOF MS. Navarro et al.56
compared HPLC to CZE (capillary zone electrophoresis), both linked to diode
array detectors, to determine the applicability of CZE to the analysis and
authentication of cranberry in fruit, juice, and extract forms. CZE was shown
to be complementary to HPLC in this report and may be an alternative approach
for some analytical groups. A general note about HPLC
columns may be helpful to readers. Some of the more recent articles reviewed
herein used core-shell columns for the HPLC analyses; the authors of those
articles noted that better resolution and peak shapes were obtained with these
columns, compared to conventional packed columns. One might hypothesize that
such column architecture lends itself to partition chromatography, rather than
adsorption mechanisms. Readers less familiar with
cranberry analysis would benefit from first studying the AHP monograph on
cranberry,8 since it reviews most of the studies and methods listed
in Table 1, with the exception of the very recent (2018) publications. 10.
Conclusions There is a growing body of
reliable data indicating that cranberry juice and extract products are
frequently adulterated. Possibly driven by supply/demand issues and/or
financial incentives, such fraudulent products likely deprive consumers of the perceived
and documented health benefits of cranberry. A number of analytical
methods are reviewed in this guidance document, with the seemingly most broadly
applicable and useful of those highlighted for the benefit of readers. In
general, methods most useful for checking juices for quality and lack of
adulteration include analyses for organic acids (HPLC-RI or UV) sugars (HPLC-RI
or 12C/13C ratios by MS) and anthocyanin pigments
(HPLC-Vis). For fruits and fruit-derived extracts and powders, the higher
resolution separation techniques like HPTLC and HPLC/UPLC give better
separation of the complex mixtures present. HPLC would need to be coupled to a
specific detection methodology, like Vis (anthocyanins), FD (procyanidins), or
MS (all compounds). HPTLC-MS systems have been developed to address this and
other challenges. It should be noted that
none of the adulterating materials, whether they be other fruit juices or
exogenous substances, represent an apparent safety concern to consumers,
although the possible presence of peanut allergens from peanut skins could be
of concern to a subset of the general population. *
The terms proanthocyanidin and procyanidin seem to be used interchangeably in
the literature. However, proanthocyanidin is a generic term for a family of
structurally related polyphenolic compounds comprised of the procyanidins,
prodelphinidins, propelargonidins, etc. The different proanthocyanidin classes
are distinguished by the specific flavan-3-ol hydroxylation pattern, e.g.,
3,3’,4’,5,7-pentahydroxyflavan-3-ol in case of the procyanidins, or 3,4’,5,7-tetrahydroxyflavan-3-ol
for the propelargonidins. The name “proanthocyanidin” is derived from the fact
that these compounds produce anthocyanidins when treated with a mineral acid.
Specifically, a procyanidin will produce the anthocyanidin cyanidin, a prodelphinidin
will yield the anthocyanidin delphinidin, a propelargonidin will be converted
into pelargonidin, etc.
†
According
to the International Union of Pure and Applied Chemistry (IUPAC), the term
“oligomer” is defined as a substance composed of a few molecules repetitively
linked to each other. The addition of another unit leads to a notable change in
the physical properties of the molecule. While there is no universally accepted
number of flavan-3-ol units that make up an oligomeric PAC, for the purpose of
this document, the term “oligomer” describes PACs having 3-10 units.
‡ Several abbreviations for
the same molecule can be found in the literature. Procyanidin A2 is written as
PAC-A2 by Boudescoque et al.,51 and Boudescoque-Delaye et
al.,52 as A2 or procyanidin A2 by Bakhytkyzy et al.,57 or
as ProA2 by Krueger et al.49 Similar inconsistencies occur for other
procyanidins. For this laboratory guidance document, the terminology by Krueger
et al.49 has been followed.
11. References- Brendler T, Gafner S. Adulteration
of Cranberry (Vaccinium macrocarpon) –
Botanical Adulterants Bulletin. Austin, TX: ABC-AHP-NCNPR Botanical
Adulterants Prevention Program; Botanical Adulterants Bulletin. 2017;1-8.
- Thesaurus
of Agricultural Organisms: Pests, Weeds and Diseases. Volume One: A to M. New York:
Chapman and Hall/CRC Press; 1990.
- Goetz P, Ghedira K. Phytothérapie
Anti-infectieuse. Paris:
Springer-Verlag; 2012.
- United States Department of
Agriculture (USDA), Agricultural Research Service (ARS), National Genetic
Resources Program. Germplasm Resources
Information Network (GRIN) Online Database. Beltsville, MD: National
Germplasm Resources Laboratory.
http://www.ars-grin.gov. Accessed 24 October 2018.
- Murray MT, Pizzorno JE. The Encyclopedia of Healing Foods. New York: Atria Books. 2005: 268-271.
- Flora of China. eFloras.org
website. http:/www.efloras.org. Accessed
August 21, 2018.
- Oxycoccus. Wikipedia database. https:/it.wikipedia.org/wiki/ghorn. Accessed
August 21, 2018.
- Upton R, Brendler T, eds. American Herbal
Pharmacopoeia and Therapeutic Compendium: Cranberry Fruit: Vaccinium
macrocarpon Aiton. Scotts Valley, CA: American Herbal Pharmacopoeia.
Monograph revision; 2016.
- McGuffin M, Kartesz JT,
Leung AY, Tucker AO. Herbs of Commerce.
2nd ed. Silver Spring, MD:
American Herbal Products Association; 2000.
- Vander Kloet SP. The Genus Vaccinium in North America. Ottawa: Agriculture Canada. 1988: 107-118.
- Upton R, Graff A, Jolliffe
G, Länger R,
Williamson E, eds.
American
Herbal Pharmacopoeia: Botanical Pharmacognosy—Microscopic Characterization of
Botanical Medicines. Boca
Raton, FL: CRC Press; 2011: 689-691.
- Khaneja
M, Gupta S, Sharma A. Pharmacognostical and preliminary phytochemical
investigations on fruit of Vaccinium
macrocarpon Aiton. Pharmacogn J.
2015;7:333-338.
- Schlautman B, Bolivar-Medina J, Hodapp S, Zalapa J. Cranberry SSR multiplexing panels for DNA
horticultural fingerprinting and genetic studies. Scientia Hort. 2017;219:280-286.
- Schlautman B, Fajardo D, Bougie T, Wiesman E, Polashock
J, Vorsa N, Steffan S, Zalapa J. Development and validation of 697 novel
polymorphic genomic and EST-SSR markers in the American cranberry (Vaccinium macrocarpon Ait.). Molecules. 2015;20:2001-2013.
- Fajardo D, Morales J, Zhu H, Steffan, S, Harbut R, Bassil
N, Hummer K, Polashock J, Vorsa N, Zalapa J. Discrimination of
American cranberry cultivars and assessment of clonal heterogeneity using
microsatellite markers. Plant Mol
Biol Rep. 2013;31:264-271.
- Georgi L, Johnson-Cicalese J, Honig J, Das SP, Rajah VD,
Bhattacharya D, Bassil N,
Rowland LJ, Polashock J, Vorsa N. The
first genetic map of the American cranberry: exploration of synteny
conservation and quantitative trait loci. Theor Appl Genetics. 2013;126:673-692.
- Zhu H, Senalik D, McCown BH, Zeldin EL, Speers J, Hyman
J, Bassil N, Hummer K, Simon PW, Zalapa JE. Mining and validation of pyrosequenced simple sequence repeats (SSRs)
from American cranberry (Vaccinium
macrocarpon Ait.) Theor Appl
Genetics. 2012;124:87-96.
- Cesoniene L, Daubaras R, Paulauskas A,
Zukauskiene J, Zych M. Morphological
and genetic diversity of European cranberry (Vaccinium oxycoccus L., Ericaceae) clones in Lithuanian reserves. Acta
Soc Bot Poloniae.
2013;82:211-217.
- Herbst N, Wilson T, Klein
J, Cooper S. Detection of cranberry and blueberry (Vaccinium spp.) DNA by PCR amplification of the MatK gene. FASEB J. 2014;28(1 Supplement):LB386.
- Howell AB, Reed
JD, Krueger CG, Winterbottom R, Cunningham DG, Leahy M. A-type cranberry
proanthocyanidins and uropathogenic bacterial anti-adhesion activity. Phytochemistry. 2005;66:2281-2291.
- Appeldoorn MM, Vincken J-P,
Sanders M, Hollman PCH, Gruppen H. Combined normal-phase and reversed-phase
liquid chromatography/ESI-MS as a tool to determine the molecular diversity of
A-type procyanidins in peanut skins. J
Agric Food Chem. 2009;57:6007-6013.
- Weseler AR, Bast A.
Masquelier’s grape seed extract: from basic flavonoid research to a
well-characterized food supplement with health benefits. Nutr J. 2017; 6 Article #5,19 pp.
- Weber HA, Hodges AE,
Guthrie JR, O'Brien BM, Robaugh D, Clark AP, Harris RK, Algaier JW, Smith CS.
Comparison of proanthocyanidins in commercial antioxidants: Grape seed and
pine bark extracts. J Agric Food Chem. 2007;55:148-156.
- La VD, Bergeron C,
Gafner S, Grenier D. Grape seed extract suppresses lipopolysaccharide‐induced matrix
metalloproteinase (MMP) secretion by macrophages and inhibits human MMP-1 and
-9 activities. J Periodontol. 2009;80:1875-1882.
- Monagas M,
Hernández-Ledesma B, Garrido I, J Martín-Alvarez P, Gómez-Cordovés C, Bartolomé
B. Quality assessment of commercial dietary antioxidant products from Vitis vinifera L. grape seeds. Nutr Cancer. 2005;53:244-254.
- O’Keefe SF, Wang H.
Effects of peanut skin extract on quality and storage stability of beef
products. Meat Sci. 2006;73:278-286.
- Constanza KE, White
BL, Davis JP, Sanders TH, Dean LL. Value-added processing of peanut skins:
Antioxidant capacity, total phenolics, and procyanidin content of spray-dried
extracts. J Agric Food Chem. 2012;60:10776-10783.
- Dudek MK, Gliński VB,
Davey MH, Sliva D, Kaźmierski S, Gliński JA. Trimeric and tetrameric A-type
procyanidins from peanut skins. J Nat
Prod.2017;80:415-426.
- Bansode RR, Randolph
P, Ahmedna M, Hurley S, Hanner T, Baxter SA, Johnston TA, Su M, Holmes BM, Yu
J, Williams LL. Bioavailability of polyphenols from peanut skin extract
associated with plasma lipid lowering function. Food Chem. 2014;148:24-29.
- Kim SM, Kang S-W,
Jeon J-S, Um B-H. A comparison of Pycnogenol® and bark extracts from
Pinus thunbergii and Pinus densiflora: Extractability,
antioxidant activity and proanthocyanidin composition. J Med Plants Res. 2012;6:2839-2849.
- Navarrete P, Pizzi A,
Pasch H, Rode K, Delmotte L. MALDI-TOF and 13C NMR characterization
of maritime pine industrial tannin extract. Ind
Crops Prod. 2010;32:105-110.
- Jerez M, Pinelo M,
Sineiro J, Núñez MJ. Influence of extraction conditions on phenolic yields from
pine bark: assessment of procyanidins polymerization degree by thiolysis. Food Chem. 2006;94:406-414.
- Bianchi S, Kroslakova
I, Janzon R, Mayer I, Saake B, Pichelin F. Characterization of condensed
tannins and carbohydrates in hot waterbark extracts of European softwood
species. Phytochemistry. 2015;120:53-61.
- Villani TS, Reichert
W, Ferruzzi MG, Pasinetti GM, Simon JE, Wu Q. Chemical investigation of
commercial grape seed derived products to assess quality and detect
adulteration. Food Chem. 2015;170:271-280.
- Labarbe B, Cheynier V,
Brossaud F, Souquet J-M, Moutounet M. Quantitative fractionation of grape
proanthocyanidins according to their degree of polymerization. J Agric Food Chem. 1999;47:2719-2723.
- Oszmiański J, Wolniak
M, Wojdyło A, Wawer I. Influence of apple pure´e preparation and storage on
polyphenol contents and antioxidant activity. Food Chem. 2008;107:1473-1484.
- Pastene E, Troncoso
M, Figueroa G, Alarcón J, Speisky H. Association between polymerization degree
of apple peel polyphenols and inhibition of Helicobacter
pylori urease. J Agric Food Chem. 2009;57:416-424.
- Jiang X, Liu Y, Wu Y,
Tan H, Meng F, Wang YS, Li M, Zhao L, Liu L, Qian Y, Gao L, Xia T. Analysis of
accumulation patterns and preliminary study on the condensation mechanism of
proanthocyanidins in the tea plant [Camellia
sinensis]. Sci Rep. 2015;5:8742-8756.
- Sarnoski PJ, Johnson
JV, Reed KA, Tanko JM, O’Keefe SF. Separation and characterisation of
proanthocyanidins in Virginia type peanut skins by LC–MSn. Food Chem. 2012;131:927-939.
- Prieur C, Rigaud J,
Cheynier V, Moutounet M. Oligomeric and polymeric procyanidins from grape
seeds. Phytochemistry. 1994;36:781-784.
- Sun B, Leandro C,
Ricardo da Silva JM, Spranger I. Separation of grape and wine proanthocyanidins
according to their degree of polymerization. J Agric Food Chem. 1998;46:1390-1396.
- Spranger I, Sun B,
Mateus AM, Freitas Vd, Ricardo-da-Silva JM. Chemical characterization and
antioxidant activities of oligomeric and polymeric procyanidin fractions from
grape seeds. Food Chem. 2008;108:519-532.
- European Fruit Juice
Association. Reference guidelines for cranberry juice:
http://www.aijn.org/publications/code-of-practice/individual-reference-guidelines.
Accessed September 17, 2018. [Note: must be a subscriber to accept the
document(s)].
- Commodity specifications
for bottled juices. United States Department of Agriculture. June 2014.
https://www.ams.usda.gov/sites/default/files/media/Commodity%20Specification%20for%20Bottled%20Juices%2C%20June%202014.pdf,
pp 11-12. Accessed October 11, 2018.
- USP
41-NF 36. Rockville, MD: United States Pharmacopeial Convention;
2018:4554-4555.
- Hong V, Wrolstad RE. Cranberry juice composition. J AOAC Int. 1986;69:199-207.
- Hong V, Wrolstad RE.
Detection of adulteration in commercial cranberry juice drinks and concentrates.
J AOAC Int. 1986;69:208-213.
- Prior RL, Fan E, Ji H, Howell A, Nio C, Payne MJ, Reed J. Multi‐laboratory validation of a standard
method for quantifying proanthocyanidins in cranberry powders. J Sci Food Agric. 2010;90:1473-1478.
- Krueger CG, Chesmore N, Chen X, Parker J, Khoo C, Marias
JPJ, Shanmuganayagam
D, Crump P, Reed JD. Critical reevaluation of the 4-(dimethylamino)
cinnamaldehyde assay: cranberry proanthocyanidin standard is superior to
procyanidin A2 dimer for accurate quantification of proanthocyanidins in
cranberry products. J Funct Foods.
2016;22:13-19.
- Sintara M, Li L, Cunningham DG, Prior RL, Wu X, Chang T.
Single-laboratory validation for determination of total soluble
proanthocyanidins in cranberry using 4-dimethylaminocinnamaldehyde. JAOAC Int. 2018;101:805-809.
- Boudesocque L, Dorat J, Pothier J, Gueiffier A,
Enguehard-Gueiffier C. High-performance thin-layer chromatography-densitometry:
A step further for quality control of cranberry extracts. Food Chem. 2013;136:866-871.
- Boudesocque-Delaye L, Arnaud Lanoue A, Dorat J, Bruyère
F, Gueiffier A, Enguehard-Gueiffier C. Quality control of commercial cranberry
products: HPTLC-densitometry a new deal. Food
Control. 2018;86:214-223.
- Brown PN, Shipley PR.
Determination of anthocyanins in cranberry fruit and cranberry fruit products
by high-performance liquid chromatography with ultraviolet detection:
single-laboratory validation. J AOAC Int.
2011;94:459-466.
- Gao C, Cunningham DG, Liu
H, Kho C, Gu L. Development of a thiolysis HPLC method for the analysis of
procyanidins in cranberry products. J
Agric Food Chem. 2018;66:2159-2167.
- Puigventos L, Nuñez O,
Saurina J. HPLC fingerprints for the authentication of cranberry-based products
based on multivariate calibration approaches. Curr Anal Chem. 2017;13:256-261.
- Navarro M, Nuñez O, Saurina J, Hernández-Cassou S, Puignou
L. Characterization of fruit products by capillary zone electrophoresis and
liquid chromatography using the compositional profiles of polyphenols:
application to authentication of natural extracts. J Agric Food Chem. 2014;62:1038-1046.
- Bakhytkyzy I, Nuñez O, Saurina J. Determination of flavanols by
liquid chromatography with fluorescence detection. Application to the
characterization of cranberry-based pharmaceuticals through profiling and
fingerprinting approaches. J Pharm Biomed
Anal. 2018;156:206-213.
- Prior RL, Lazarus SA, Cao G, Muccitelli H, Hammerstone
JF. Identification of procyanidins and anthocyanins in blueberries and
cranberries (Vaccinium spp.) using
high-performance liquid chromatography/mass spectrometry. J Agric Food Chem. 2001;49:1270-1276.
- Gu L, Kelm MA, Hammerstone JF, Beecher G, Holden J,
Haytowitz D, Prior RL. Screening of foods containing proanthocyanidins and
their structural characterization using LC-MS/MS and thiolytic degradation. J Agric Food Chem. 2003;51:7513−7521.
- Sánchez-Patán F, Bartolomé B, Martín-Alvarez PJ,
Anderson M, Howell A, Monagas M. Comprehensive assessment of the quality of
commercial cranberry products. Phenolic characterization and in vitro
bioactivity. J Agric Food Chem. 2012;60:3396-3408.
- Feliciano RP, Krueger CG, Shanmuganayagam D, Vestling MM, Reed JD. Deconvolution of matrix-assisted laser
desorption/ionization time-of-flight mass spectrometry isotope patterns to
determine ratios of A-type to B-type interflavan bonds in cranberry
proanthocyanidins. Food Chem. 2012;135:
485–1493.
- Jungfer E, Zimmermann
BF, Ruttkat A, Galensa R. Comparing procyanidins in selected Vaccinium species by UHPLC-MS2 with regard to authenticity
and health effects. J Agric Food Chem. 2012;60:9688-9696.
- Barbosa S, Pardo-Mates N, Hidalgo-Serrano M, Saurina J, Puignou L, Núñez O. Detection and quantitation of
frauds in the authentication of cranberry-cased extracts by UHPLC-HRMS
(Orbitrap) polyphenolic profiling and multivariate calibration methods. J Agric Food Chem. 2018;66:9353-9365.
- Horwitz, W. AOAC Guidelines for single laboratory
validation of chemical methods for dietary supplements and botanicals; AOAC
International: Gaithersburg, MD, 2002.
- Zhang Y, Krueger D, Durst
R, Lee R. Wang D, Seeram N, Heber D. International multidimensional
authenticity specification (IMAS) algorithm
for detection of commercial pomegranate juice adulteration. J Agric Food Chem. 2009;57:2550-2557.
- Thompson RS, Jacques D, Haslam E, Tanner RJN. Plant
proanthocyanidins. Part I. Introduction; the isolation, structure, and
distribution in nature of plant procyanidins. J Chem Soc Perkin Trans I. 1972;1387-1399.
- Torres J, Selga A. Procyanidin size and composition by
thiolysis with cysteamine hydrochloride and chromatography. Chromatographia. 2003;57:441-445.
- Ye L, Neilson A, Sarnoski P, Ray WK, Duncan S, Boyer R,
O’Keefe SF. Comparison of A-type proanthocyanidins in cranberry and peanut skin
extracts using matrix assisted laser desorption ionization-time of flight mass
spectrometry. J Mol Genet Med.
2016;10(2):1000209.
|