By John H. Cardellina II, PhD*
Keywords:
Citrus
paradisi, Rutaceae,
grapefruit seed extract, GFSE, adulterant, adulteration, benzalkonium chloride,
benzethonium chloride, triclosan
CONTENTS
1. Purpose
2. Scope
3. Common and
Scientific Names
3.1 Common name
3.2 Other common
names
3.3 Accepted Latin
binomial
3.4 Synonyms
3.5 Botanical
family
4. Botanical
Description
5.
Identification and Distinction using Macroanatomical
Characteristics
6.
Identification and Distinction using Microanatomical
Characteristics
7.
Genetic Identification and Distinction
8.
Chemical Identification and Distinction
8.1
Chemistry of Citrus paradisi and potential
adulterants
Figure 1. Structures of the principal
disinfectants/microbicides found in products labeled
"Grapefruit Seed Extract"
Figure 2. Structures of naringenin and limonin,
representative of the flavanones and limonoids,
respectively, present in authentic grapefruit seed
8.2 Laboratory
methods
Table 1. Summary comparison of different approaches to
determine adulterants in GFSE
Table 2. Time sequence of the detection of adulterants
in GFSE products
8.2.1 TLC
Figure 3: HPTLC analysis of grapefruit seed
extract
8.2.2 HPLC-UV
8.2.3 HPLC-MS
8.2.4 UHPLC-UV-MS
8.2.5 GC-MS
8.2.6 1H-NMR
9. Conclusion
10. References
1.
Purpose
The case of synthetic microbicides marketed as grapefruit seed extract
(GFSE) differs from the other botanicals addressed thus far by the Botanical
Adulterants Program (BAP), in that the adulteration does not consist of
substitution by or inclusion of other botanicals, but rather the inclusion
of one or more synthetic microbicidal compounds (disinfectants) in the
products. Therefore, this Guidance Document presents a review of the analytical
technologies used to differentiate and identify the various microbicides that
have been reported from commercial GFSE products, as well as methods to
separate and identify natural grapefruit seed constituents.
2.
Scope
We are unaware of any
pharmacopoeial standards or monographs for grapefruit seed extract. In addition,
some of the commercial GFSE products are reportedly prepared from dried, ground
seeds that are boiled in water and distilled; that distillate is then treated
with ascorbic acid, hydrochloric acid, and ammonium chloride under heat and
pressure; this treatment purportedly produces microbicidal compounds resembling
benzethonium chloride from the flavonoids in the grapefruit seeds.1 However,
there are no known natural or synthetic chemical pathways whereby the natural
constituents of grapefruit seed could be transformed into such compounds, using
the reagents listed above under the conditions described. It simply defies the
logic and state of our knowledge of synthetic organic chemistry and
biosynthesis. More interestingly, the majority of published analyses of GFSE
products report only man-made microbicidal compounds and no compounds typical
of grapefruit or any citrus fruit (e.g., flavonoids, limonoids, or essential
oils).
Complicating the
selection of an analytical method is the observation that the microbicidal
compounds detected in GFSE products have changed over time. Therefore, the
ideal analytical method should be flexible enough to detect and quantify not
only any and all microbicides previously found in GFSE, but perhaps also any
other similar, commercially available microbicidal compounds.
The recommendation of
a specific method or methods in this Laboratory Guidance Document for testing
GFSE materials does not take away the responsibility of laboratory personnel to
demonstrate adequate method performance in their own laboratory using accepted
protocols outlined in the 21 CFR Part 111 and by AOAC International, ISO, WHO,
and ICH.
3. Common and
Scientific Names
3.1 Common name: grapefruit
seed extract (GFSE)
3.2 Other common names:
French: Extrait de pépins
de pamplemousse
German: Grapefruitkern Extrakt
Italian: Estratto di semi di pompelmo
Spanish: Extracto de semilla de pomelo
3.3 Accepted
Latin binomial: Citrus
paradisi Macfad.2
3.4
Synonyms: Citrus x
paradisi3
3.5 Botanical family:
Rutaceae
4. Botanical
Description
Grapefruit, Citrus
paradisi,
is believed to have originated in Barbados as a hybrid
of sweet orange (C. sinensis) and shaddock (C.
grandis); both of those species had
been introduced to Barbados from southern Asia in the
seventeenth century. Grapefruit
was first described in 1750, but was not distinguished
botanically from pomelo
until the 1830s.4 The fruit is large,
compared to other citruses,
and is characterized by a sour/acidic to semi-sweet
taste.
5.
Identification and Distinction using Macroanatomical
Characteristics
The dried seeds of Citrus
paradisi
are not easily distinguished from other Citrus
spp. seeds on a macroanatomical basis, although they are
generally larger than
most other citrus seeds, certainly lemon and lime. Like
all citrus seeds, they
are white, with a thin shell over a pith layer
protecting a seed kernel.
6.
Identification and Distinction using Microanatomical
Characteristics
No report of
microanatomical distinction of grapefruit seeds from
seeds
of other Citrus spp. was found.
7.
Genetic Identification and Distinction
Only one paper describes
the use of DNA analysis to differentiate 38
grapefruit and 3 pomelo (Citrus maxima)
samples by RAPD and SSR markers.5 In that
study, only two grapefruit
samples clustered closely with the pomelos, and the
remainder were subdivided
into three closely related groups. However, DNA analysis
would not be useful in
the case of GFSE, since the process of preparing much of
the marketplace product
uses heat, pressure, and acid treatments, very likely
decomposing the DNA of
the seed material. DNA analyses would have to be
conducted on the untreated raw
material. Here the presence of grapefruit seed, relative
to some other seed raw
material, could be confirmed, but this would have no
bearing on the presence of
synthetic microbicides in the final products.
8.
Chemical Identification and Distinction
There
is minimal information in the literature on
differentiating grapefruit seeds
from those of other citrus species. There is one report
of the application of
HPLC to distinguish the extracts of seeds of four citrus
varieties – ruby red
grapefruit, sour orange (C. aurantium),
Nova tangerine, and Cleopatra mandarin.6
However, the latter two
varieties were shown by this analysis to be the same
species, C. reticulata. Nonetheless, the method
did allow differentiation of grapefruit seed extract
from those of the other
citrus seeds examined; not surprisingly, the two
different C. reticulata samples were not easily
distinguished from one
another.
The very recent paper
by Avula et al.
7 reports improvement and
expansion of a previously
reported UHPLC-UV-MS method,
8 whereby the
reported modifications
make it possible to resolve and identify not only the
suspected adulterant
microbicides, but also the limonoids and flavonoids expected
in a true extract
of grapefruit seed. Thus, one should now be able to look at
a GFSE product with
one analytical method to examine whether it is made from
grapefruit seeds or
other citrus (e.g., lemon and orange seeds are abundantly
available as byproducts
of the juice industry) and/or whether it contains any
adulterating synthetic
microbicides.
8.1
Chemistry of Citrus paradisi and
potential adulterants
The secondary metabolites
of grapefruit seeds are predominately limonoids and
flavonoids, both being
bitter. Naringin, a diglycoside of the common flavanone
naringenin (8), is far and away the dominant
flavonoid
in grapefruit seeds. Limonoids are a unique subset of
triterpenes in which the conventional
triterpene skeleton is significantly oxidized and
cleaved in one or more
places. Seven limonoids, along with seven limonoid
glycosides (a sugar attached
to the triterpene core), have been reported from
grapefruit seeds.9-16
Limonin (9), the most abundant of grapefruit
seed limonoids, comprises ~0.5% of the dry weight of the
seeds,15
and the total limonoid content could approach
1%.16
It is noteworthy that
none of the published analyses of commercial grapefruit seed
extracts have
indicated the presence of either limonoids or flavonoids in
those products,
even though limonoid glycosides have been isolated from
citrus seeds extracted
with aqueous acid in the presence of
pectinase.
17-19 Instead, a
series of 13 analyses of commercial GFSE products over a
span of more than two
decades has revealed the presence of a number of synthetic
microbicides, shown
in Figure 1. Any commercially available quaternary ammonium
salt with at least
one lipophilic (hydrophobic) ring or chain could conceivably
come into play as
an adulterant in purported GFSE products. This class of
compounds exerts its
considerable microbicidal effect by lysing cell membranes.
For comparison
purposes, Figure 2 illustrates the structures of
8
and
9, representative
of the flavanones and limonoids, respectively, present in
grapefruit seed.
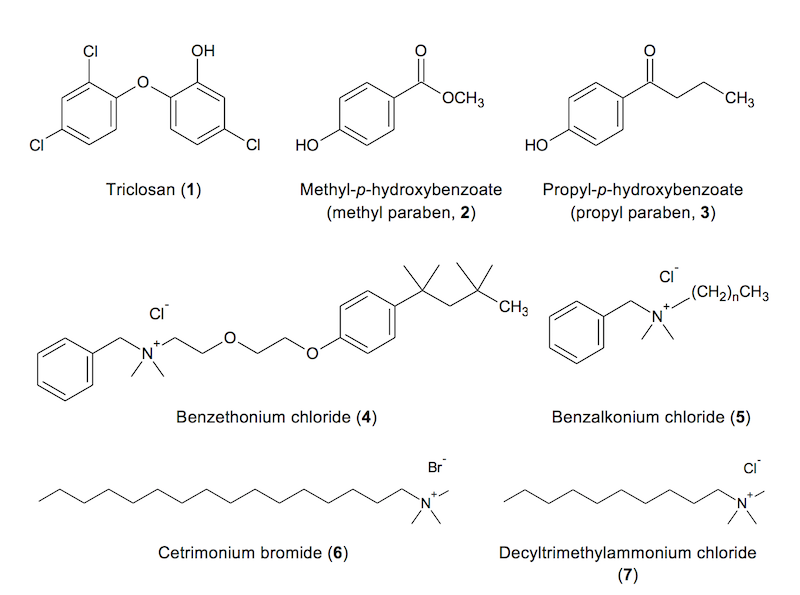
Figure 1. Structures of the
principal disinfectants/microbicides found in products labeled “Grapefruit Seed
Extract”
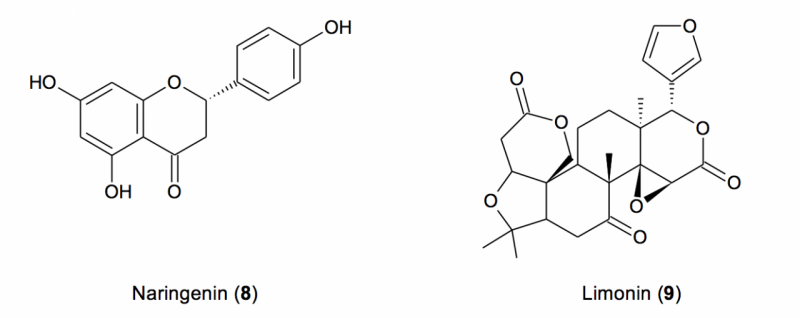
Figure 2. Structures of
naringenin and limonin, representative of the flavanones and limonoids,
respectively, present in authentic grapefruit seed
8.2 Laboratory methods
Table 1 lists the different analytical methods used to
analyze
commercial GFSE products for adulteration and considers
the key advantages and
disadvantages of each technique.
Table 1. Summary comparison of different approaches
to determine adulterants in GFSE
|
Method
|
Applicable to
|
Pro
|
Contra
|
|
TLC
|
liquid or powder products
|
quick, inexpensive
basic systems
affordable for smaller labs
reference compounds
available
|
high-end equipment
expensive
more qualitative
than quantitative
some products (e.g.,
glycerin extracts) may require time-consuming sample preparation
|
|
HPLC-UV
|
liquid or powder products
|
standard equipment
in many laboratories
most compounds of
interest have chromophores
reference compounds
available
|
equipment is costly
some of the synthetic
microbicides do not have a chromophore
|
|
HPLC-MS
HPLC-ESIMS
|
liquid or powder products
|
equipment
increasingly common in laboratories
reference compounds
available
|
equipment is very
costly
|
|
HPLC-UV-MS
|
liquid or powder products
|
equipment
increasingly common in laboratories
reference compounds
available
|
equipment is very
costly
|
|
GC-MS
|
liquid or powder product
|
standard equipment
in many laboratories
reference compounds
available
unknown
microbicides may be identified using commercially available libraries
|
equipment is costly
some products (e.g.,
glycerin extracts) may require time-consuming sample preparation
|
|
1H-NMR
|
liquid or powder products
|
reference compounds
available
|
equipment is very
costly
sensitivity is
lower compared to other methods
|
Complicating the evaluation of the various
published analytical methods
for GFSE adulteration and potential selection of a
method to use is the fact
that the adulterant microbicides found in GFSE
commercial products changed over
time. Table 2 summarizes the
chronological record of the appearance of the various
GFSE adulterant microbicides.
Table 2. Time sequence of the detection of
adulterants in GFSE products
|
year, first author and ref
|
1a
|
2a
|
3a
|
4a
|
5a
|
6a
|
7a
|
|
1991 Nishimia et al.20
|
√
|
√
|
|
|
|
|
|
|
1996 Sakamoto et al.21
|
√
|
√
|
|
|
|
|
|
|
1999 von Woedtke et al.22
|
√
|
√
|
|
√
|
|
|
|
|
2001 Takeoka et al.23
|
|
|
|
√
|
|
|
|
|
2001 Terreaux et al.24
|
|
|
|
√
|
|
|
|
|
2004 Spitaler et al.25
|
|
√
|
√
|
√
|
√
|
|
|
|
2005 Takeoka et al.26
|
|
|
|
|
√
|
|
|
|
2006 Ganzera et al.27
|
|
√
|
√
|
√
|
√
|
|
|
|
2007 Avula et al.8
|
√
|
|
|
√
|
|
|
|
|
2007 Spinosi et al.28
|
|
|
|
√
|
|
√
|
√
|
|
2008 Sugimoto et al.29
|
|
|
|
√
|
√
|
|
|
|
2008 Bekiroglu et al.30
|
|
|
|
√
|
|
|
|
|
2016 Avula et al.7
|
|
|
|
√
|
|
|
|
The 2001 paper by Terreaux
et al.24 was not included in our
original review of the adulteration of
GFSE,31 because it was not uncovered in
several literature searches. Terreaux et al. reported an
HPLC-UV analysis
of 17 commercial GFSE products, 9 of which contained 4.
Six of those samples had
high levels of this adulterant, 6.7-20.4%.
8.2.1 TLC
The method of von Woedtke
et al.22 was evaluated in this review.
Comments: Both
UV and colorimetric approaches
were used for determining the presence of the adulterant
microbicides, but
their concentration could only be estimated by
comparison of spot intensity to different
concentrations of reference standards. However, this
relatively inexpensive and
rapid analysis can qualitatively distinguish grapefruit
seed components from
synthetic microbicides quite readily, providing a quick
yes/no answer regarding
adulteration. Further analyses might be necessary to
assess more thoroughly the
quality of the material being analyzed.
Note: An example of a
HPTLC analysis of GFSE is shown in Figure 3.
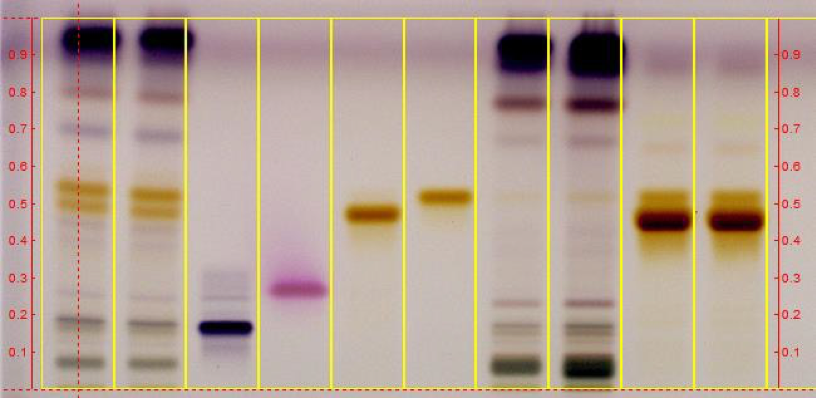
Figure 3: HPTLC analysis of
grapefruit seed extract. Image provided by Nature's Way
Brands Inc. (Green Bay, WI)
Lanes 1,2: Grapefruit
pectin; lane 3: escin; lane 4: benzethonium chloride;
lane 5; hesperidin; lane
6: naringin; lanes 7,8: grapefruit seed, reference
material; lanes 9,10:
commercial grapefruit seed extract
Stationary
phase: Silica gel 60, F254, HPTLC plates.
Concentrations:
Extracts: 100 mg/mL; Pure compounds: 1 mg/mL
Application
volume: 5 μL
Mobile phase: n-Butanol:
water: acetic acid (5:4:1) (v/v/v)
Detection: Anisaldehyde reagent, observation
under white light
8.2.2
HPLC-UV
Methods described in the
following literature were evaluated in this review:
Terreaux et al.,24
Spitaler et al.,25 and Avula et
al.8
Comments: Since
all but two
of the reported adulterant microbicides (including the most
commonly
encountered ones) from GFSE have strong aromatic
chromophores and reference
standards for all those compounds are available, HPLC-UV can
be employed for
both qualitative and quantitative analyses of the most
frequently observed adulterants.
It should be noted that, while
6 and
7 have only been
reported once from
GFSE products, they might well have evaded detection in any
UV-based analytical
method (and might not easily be discerned by NMR, either,
depending on the
complexity of the sample being analyzed).
8.2.3
HPLC-MS
Methods, including HPLC-MS,
HPLC-ESIMS and HPLC-ESIMS/MS, described in the following
literature were
evaluated in this review: Sakamoto et al.,21
Takeoka et al.,23,26
Ganzera et al.,27 and Sugimoto.29
Comments: All of these
methods used reversed phase HPLC coupled to a mass
spectrometer, most often
positive ion ESI (electrospray ionization), to confirm the
identity of and
quantify the various adulterants, after using UV detection
in separate HPLC
analyses to determine which adulterants were present. This
method offers the
added advantage of being able to detect those microbicides
that do not have a
chromophore; in fact, the quaternary ammonium microbicides
are already ionized
and readily detected by MS in the positive ion mode.
8.2.4
UHPLC-UV-MS
The recently reported
method of Avula et al.7 was evaluated in this
review.
Comments: This study, the
most recent publication in the series of 13 analyses, is the
first to provide a
method for simultaneous detection and quantification of both
the expected
limonoids and flavonoids known to occur naturally in
grapefruit seeds and also
the adulterant synthetic microbicides that have been all too
frequently
observed in commercial GFSE products.
8.2.5
GC-MS
The method described in
the following literature was evaluated in this review:
Spinosi et al.26
Comments: This study only
looked at three adulterant microbicides (
5-7)
as part of an investigation of supposed organic treatments
(GFSE) for diseases
of honeybees. GC-MS should be a sensitive and effective
method for detection of
any of the quaternary ammonium compounds, the various
parabens and even
1; all
are relatively volatile, and the
quaternary compounds are already ionized. It should be noted
that this is an
excellent method for detecting synthetic microbicides like
6 and
7, which lack a UV
chromophore; MS is the most effective detector for this type
of adulterant, and
might be the reason the authors detected these compounds,
which had not been
reported in any of the other investigations prior to 2007. A
further benefit of
GC-MS is that the mass spectral analysis (ECI-MS) gives a
richer fragmentation
pattern than other ionization methods, allowing mass
spectral library matching
of the mass spectra produced.
8.2.6 1H-NMR
Methods described in the
following literature were evaluated in this review:
Takeoka et al.23,26
and Bekiroglu et al.30
Comments: Takeoka
et al. used NMR only to
confirm the structure of 423
and 5,26
while Bekiroglu
et al. validated a quantitative 1H-NMR method
for the detection and quantitative
analysis of 4 in
GFSE products. In
their work, Bekiroglu et al. found that the limit of
quantification exceeded 20
mg/mL, based on the high signal to noise ratio of the
NMR data. They also found
that three different operators running 6 analyses on the
same sample
preparation obtained standard deviations of 0.8, 0.9 and
1.3 mg/mL,
respectively, in line with previous reports that the
handling of NMR data by
operators is the highest impact factor of influence on
the quality of a qNMR
analysis.32
9.
Conclusion
None of the published
methods has been evaluated for the detection and
quantification of all the
known adulterants of GFSE, but the most recent
contribution by Avula et al. is
the most inclusive.7 This is likely due to
the continuing change in
the composition and content of microbicidal compounds in
commercial GFSE
products over time (see Table 2). The HPLC-UV methods of
Avula et al.8
and Ganzera et al.27 have been validated,
making them attractive
methods to develop further for all the potential
adulterants. The only drawback
to this idea is that 6 and 7 (detected
by Spinosi et al.28)
do not contain a UV chromophore. That leads to the
suggestion that an HPLC-MS or
GC-MS method might be the most appropriate approach for
analyzing GFSE products
for any of the known or suspected adulterants.
Fortunately, reference standards
are available for all the potential adulterants,
facilitating development and
validation of an analytical method.
In response to reviewer
requests, we include here information provided by commercial analytical
laboratories that offer analyses of GFSE products; two laboratories responded
to our request for such information.
One laboratory performs
both the Avula HPLC-UV method8 and an HPTLC
method for 1, 2,
4, and
5.
A
second laboratory performs an unspecified HPLC-UV method for
all the
microbicides reported herein, and also offers FTIR (Fourier
Transform Infrared)
and mass spectrometry methods.
10.
References
1. Nutriteam.com. Citricidal Grapefruit seed extract.
Available at: www.nutriteam.com/gsewhat. Accessed June 29, 2016.
2. The Plant List. Version 1.1 (September 2013). http://www.theplantlist.org. Accessed October 26, 2015.
3. McGuffin M, Kartesz JT, Leung AY, Tucker AO American
Herbal Products Association’s Herbs of
Commerce. 2nd ed. Silver Spring, MD: American
Herbal Products Association; 2000.
4. Carrington S, Forde A, Fraser H, Gilmore J. Grapefruit. A-Z
of Barbados Heritage. Oxford, UK: Macmillan Education Ltd.; 2003: 90–91.
5. Corazza-Nunes MJ, Machado MA, Nunes
WMC, Cristofani M,
Targon MLPN. Assessment of genetic
variability in grapefruits (Citrus paradisi Macf.)
and pummelos (C.
maxima (Burm.) Merr.) using RAPD and SSR
markers. Euphytica. 2002;126:169-176.
6. Vikram A, Jayaprakasha GK, Patil BS. Simultaneous
determination of citrus limonoid aglycones and glucosides by high performance
liquid chromatography. Anal Chim Acta.
2007;590:180-186.
7. Avula B, Sagi S, Wang Y-H, Wang M, Gafner S, Manthey
JA, Khan IA. Liquid chromatography-electrospray ionization mass spectrometry
analysis of limonoids and flavonoids in seeds of grapefruits, other citrus
species, and dietary supplements. Planta
Med. 2016;82:1058-1069.
8. Avula B, Dentali S, Khan IA. Simultaneous
identification and quantification by liquid chromatography of benzethonium
chloride, methyl paraben and triclosan in commercial products labeled as
grapefruit seed extract. Pharmazie.
2007;62:593-596.
9. Bennett RD. Acidic
limonoids of grapefruit seeds. Phytochemistry. 1971;10:3065-3068.
10. Bennett RD, Hasegawa S.
7α-oxygenated limonoids from the Rutaceae. Phytochemistry. 1982;21:2349-2354.
11. Hasegawa S, Bennett RD,
Herman Z, Fong CH, Ou P. Limonoid glycosides in citrus. Phytochemistry, 1989;28:1717-1720.
12. Bennett RD, Hasegawa S, Herman Z. Glucosides of
acidic limonoids from citrus. Phytochemistry. 1989;28:2777-2780.
13. Jayaprakasha GK, Brodbelt JS, Bhat, NG, Patil BS.
Rapid methods for the separation of bioactive compounds from citrus, Paper presented
at: 228th ACS National Meeting; August 22-26, 2004; Philadelphia, PA.
14. Jayaprakasha G, Patil B, Bhat N. Process for the isolation of limonoid glucosides from citrus. US Patent App. 11/696,845, 200, 2007.
15. Yu J, Dandekar DV, Toledo RT, Singh RK, Patil BS.
Supercritical fluid extraction of limonoids and naringin from grapefruit (Citrus paradisi Macf.) seeds. Food
Chem. 2007;105:1026-1031.
16. Braddock RJ, Bryan CR. Extraction parameters and
capillary electrophoresis analysis of limonin glucoside and phlorin in citrus
byproducts. J Agric Food Chem. 2001;49:5982-5988.
17. Ozaki Y, Miyake M, Maeda H, Ifuku
Y, Bennett RD, Herman Z, Fong CH, Hasegawa S.
Ichangensin glucoside in Citrus junos, Citrus
sudachi, and Citrus sphaerocarpa.
Phytotherapy. 1991;30:2659-2661.
18. Bennett RD, Miyak, M, Ozaki Y, Hasegawa S. Limonoid
glycosides in Citrus aurantium. Phytochemistry.
1991;30:3803-3805.
19. Miyake M, Ozaki Y, Ayano S, Bennett RD, Herman Z,
Hasegawa S. Limonoid glucosides in calamondin seeds. Phytochemistry.
1992;31:1044-1046.
20. Nishina A, Kihara H,
Uchibori T, Oi T. Antimicrobial substances in “DF-100”, extract of grapefruit seeds. Bokin
Bobai (J Antibact Antifung Agents). 1991;19:401-404.
21. Sakamoto S, Sato K,
Maitani T, Yamada T. Analysis of components in natural food additive
“grapefruit seed extract” by HPLC and LC/MS. Eisei Shikenjo Hokoku (Bull Natl Inst Health
Sci.). 1996;114:38-42.
22. von Woedtke T,
Schlüter B, Pflegel P, Lindequist U, Jülich W-D. Aspects of the antimicrobial
efficacy of grapefruit seed extract and its relation to preservative substances
contained. Pharmazie. 1999;54:452-456.
23. Takeoka G, Lan D,
Wong RY, Lundin R, Mahoney N. Identification of benzethonium chloride in
commercial grapefruit seed extracts. J
Agric Food Chem. 2001;49:3316-3320.
24. Terreaux C,
Chevalley I, Hostettmann K. Grapefruit seed extract: a natural antibiotic? Schweizer Apotheker Zeitung. 2001;24:823-825.
25. Spitaler R, Marschall K,
Zidorn C, Markus K, Zelger R, Stuppner H.
Apple scab control with grapefruit seed extract: no
alternative to
chemical fungicides. Prob Org Chem. 2004;
archived at http://orgprints.org/14543/.
Accessed May 2, 2017.
26. Takeoka GR, Dao LT,
Wong RY, Harden LA. Identification of benzalkonium chloride in commercial
grapefruit seed extracts. J Agric Food
Chem. 2005;53:7630-7636.
27. Ganzera M, Aberham
A, Stuppner H. Development and validation of an HPLC/UV/MS method for
simultaneous determination of 18 preservatives in grapefruit seed extract. J Agric Food Chem. 2006;54:3768-3772.
28. Spinosi V, Semprini
P, Langella V. Presence of chemical additives and microbial inhibition capacity
in grapefruit seed extracts used in apiculture. Veterinaria Italiana. 2007;43:109-113.
29. Sugimoto N, Tada A,
Kuroyanagi M, Yoneda Y, Yun YS, Kunugi A, Sato K, Yamazaki T, Tanamoto K.
Survey of synthetic disinfectants in grapefruit seed extract and its compounded
products. Shokuhin Eiseigaku Zasshi.
2008;49:56-62.
30. Bekiroglu S, Myrberg
O, Ostman K, Ek M, Arvidsson T, Rundlöf T, Hakkarainen B. Validation of a
quantitative NMR method for suspected counterfeit products exemplified on
determination of benzethonium chloride in grapefruit seed extracts. J Pharm Biomed Anal. 2008;47:958-961.
31. Cardellina JH, II.
Adulteration of commercial “grapefruit seed extract” with synthetic antimicrobial
and disinfectant compounds. HerbalGram. May-July 2012,94:62-66.
32. Malz F, Jancke H. Validation of quantitative NMR. J
Pharm Biomed Anal. 2005;38:813-823.