 Click to view or download PDF version
Click to view or download PDF version
APRIL 2018
Pomegranate Products Laboratory Guidance
Document
By John H.
Cardellina II, PhD*
*ReevesGroup,
Virginia Beach, VA 23451,
Correspondence: email
Keywords:
pomegranate,
adulteration, Punica granatum L.,
Lythraceae, Punicaceae, ellagic acid, punicalagins, punicalins, HPLC, HPLC-UV
CONTENTS1. Purpose
2. Scope
3. Common and Scientific Names
3.1 Common name
3.2 Other common names
3.3 Accepted Latin binomial
3.4 Synonyms
3.5 Botanical family
4. Botanical Description
5. Identification and Distinction Using Macroanatomical Characteristics
6. Identification and Distinction Using Microanatomical Characteristics
7. Genetic Identification and Distinction
8. Chemical Identification and Distinction
8.1 Chemistry of Punica granatum and potential adulterants
Figure 1: Major polyphenols in peel and mesocarp of pomegranate fruit, according to Fischer et al.26
8.2 Laboratory methods
8.2.1 Juice
Table 1. Comparison of different analytical approaches to determine adulterants in pomegranate juice
8.2.2 Extract
Table 2. Comparison of different analytical approaches to determine adulterants in pomegranate extracts and extract-based products
9. Conclusion
10. References
1.
Purpose
Pomegranate has rapidly become
one of the most popular ‘healthy’ fruits, with an array of extracts appearing
in the botanical dietary supplement markets and a plethora of juice products in
the beverage industry. There is considerable evidence that both product
categories have been subjected to adulteration with various undeclared,
lower-cost exogenous ingredients.1 Therefore, this Laboratory Guidance
Document presents a review of the analytical technologies used to determine
whether pomegranate juice or extract products are adulterated and to identify
the adulterants involved.
2. Scope
The analytical challenge
arising from adulteration of pomegranate products is complex because different
products are adulterated in different ways. Pomegranate juice has been found to
be diluted by a variety of lower-cost, more readily-available juices, and
colorants may be added to adjust the color to approximate true pomegranate
juice more closely. Pomegranate extract products have been adulterated by
addition of exogenous ellagic acid (EA) or made entirely from unknown or
unidentified source materials, with little-to-no pomegranate constituents, but
significant amounts of EA present. The methods discussed in this guidance
document were developed for either juice or extract products, but may not be
applicable to other pomegranate food products (e.g., yogurt or jelly) or
medicinal products derived from pomegranate plant parts other than the fruit
(e.g., leaves).
The evaluation of a
specific analytical method or methods in this Laboratory Guidance Document for
testing pomegranate materials does not reduce or remove the responsibility of
laboratory personnel to demonstrate adequate method performance in their own
laboratory using accepted protocols outlined in various domestic (in the United
States) or international legal and/or regulatory documents, e.g., the 21 CFR
Part 111 (Dietary Supplement GMPs, in the US Code of Federal Regulations) and
Part 117 (Food Safety Modernization Act Final Rulemaking for Current Good
Manufacturing Practice and Hazard Analysis and Risk-Based Preventive Controls
for Human Food, in the US Code of Federal Regulations), and by AOAC
International, International Standards Organization (ISO), World Health
Organization (WHO), and the International Council on Harmonisation (ICH).
3. Common and Scientific
Names
3.1
Common name: Pomegranate
3.2
Other common names
French: grenade, pomme de grenade (Quebec
Province, Canada)
Spanish: granada
Italian: melograno
German: Granatapfel
Dutch: granaatappel
Persian: anâr (انار)
Sanskrit: dalim or dadima
3.3
Accepted Latin binomial: Punica granatum L.
3.4
Synonyms: Punica nana L.
3.5
Botanical family: Lythraceae
Note: Pomegranate was
previously classified in the botanical family Punicaceae, which has been
combined with the family Lythraceae on the basis of genetic and morphological
characteristics.2
4.
Botanical Description
Punica granatum is a fruit-bearing deciduous shrub or small
tree in the
family Lythraceae that grows between five and 10 m (16-30
feet) tall. The
pomegranate tree enjoys considerable longevity, with some specimens in France
reported to have survived two centuries. On multiple, spiny branches, the deciduous
leaves are opposite or in whorls of five or six, short-stemmed,
oblong-lanceolate, leathery, and 1-10 cm (0.4-4 in) long. Showy red, white, or
variegated flowers are found on the branch tips, singly or in clusters of up to
five flowers. Nearly round, but crowned at the base by the prominent calyx, the pomegranate fruit has a tough, leathery skin or rind, and is basically yellow overlaid with light
or deep pink or rich red. The interior is separated by membranous walls and
white spongy tissue into compartments packed with transparent sacs filled with
tart, flavorful, fleshy, juicy pulp (the aril). In each sac, there is one white
or red, angular, soft or hard seed. The arils represent about 52% of the weight
of the whole fruit.3
Pomegranate is believed
to have originated in an area encompassing what are now Iran, Afghanistan, Pakistan,
and northern India. Pomegranate has played a prominent role in Greek mythology,
symbolism and ceremonies, as well as numerous other religious beliefs,
including Buddhism, early Christianity, Hinduism, Islam, Judaism and Zoroastrianism.3-5
All parts of the
pomegranate plant (root, bark, leaves, flowers, fruit, and seeds) have been
used in Ayurvedic medicine in India for various health conditions that range
from an anti-parasitic agent and blood tonic to treatments for ulcers, canker
sores, and diabetes.5 More recently, the antitumor, antidiabetic,
cardioprotective, antioxidant, antimicrobial, anti-Alzheimer’s, anti-inflammatory,
and antiviral properties of preparations of the pomegranate fruit have received
attention.6-12
5.
Identification and Distinction Using Macroanatomical Characteristics
Fruit, a globose berry, 5–13 cm in diameter, with a
leathery rind enclosing numerous seeds (arils), angular/wedge shaped, variously
colored—yellowish green, white, reddish brown, or occasionally blackish purple.13,14
6.
Identification and Distinction Using Microanatomical Characteristics
Thorough descriptions of
the microscopic characteristics of pomegranate fruit have been published by the
World Health Organization (WHO) and The
Ayurvedic Pharmacopoeia of India.13,14 A 2015 publication
provides detailed information on the microscopical analysis of pomegranate
fruit, along with color images of the important microanatomical features of
pomegranate fruit.15 While microscopic analysis will provide
information about the authenticity and purity of cut or powdered crude
pomegranate fruit, this approach is not suitable when the typical
characteristics are absent, i.e., in juices or extracts. However, microscopy is
useful in the analysis of juice powders to ensure that they have the
characteristic features of juice powders processed in the manner declared; such
analyses can reveal added bulking agents (diluents, e.g., maltodextrin) that
may not be declared.
7.
Genetic Identification and Distinction
A 2016 study
demonstrated the use of a DNA-based method, SCAR (Sequence Characterized
Amplified Regions) analysis, to detect as low as 1% adulteration of pomegranate
juice by ten other botanical sources of anthocyanins that have been reported or
might be used as adulterants of pomegranate products. The SCAR marker selected
as a positive control for pomegranate, designated ScPg231, correctly
identified eleven different accessions of P.
granatum; moreover, it also identified pomegranate in four product mixtures
– two herbal teas (2% and 20% pomegranate, respectively), a jam containing
pomegranate, lemon, agave and pectin, and a juice mix containing 3.5%
pomegranate juice concentrate. These results indicate that relatively
short-length SCAR markers may be highly useful for identifying components with
partially degraded DNA.16
Another report17
expanded upon earlier studies18-20 of another DNA-based method, RAPD
(Random Amplified Polymorphic DNA), and demonstrated an ability to distinguish
and identify 47 Chinese cultivars of P.
granatum by application of RAPD. Screening of 60 primers revealed 11 primers
that gave reproducible polymorphic band patterns. While previous RAPD
investigations of pomegranate18-20 used a cluster analysis approach
to parse the data, the results from the primers employed in this study could be
used to generate a cluster identification diagram, wherein the first primer
selected divided the group of 47 cultivars into (+) or (-) subgroups (i.e., presence
or absence of the marker band, respectively); in turn, each primer was then
used to further divide the remaining groups in the same manner until all 47
cultivars were separated into distinct entities, based on polymorphic band
patterns. This technique would have its best application in verifying the
identity and cultivar of raw material supplies, rather than identifying
adulteration in finished products, although the appearance of unexpected
polymorphic band patterns would likely be an indication of substitution
(different cultivar than expected or stipulated) or adulteration with other
fruit. There are some well-known limitations to the RAPD methodology, in
particular low reproducibility and difficulties in the interpretation of the
results.21
8.
Chemical Identification and Distinction
8.1
Chemistry of Punica granatum and potential
adulterants
The chemistry of
pomegranate is dominated by phenolic compounds of differing complexity, from
benzoic acid (simple) to anthocyanins (complex) to gallotannins and
ellagitannins (very complex).7 The secondary metabolites of
pomegranate are far from fully identified; a recent investigation of the outer skin,
inner skin, and divider membranes revealed a total of 79 phenolic compounds,
thirty of which had not been reported previously, including proanthocyanidins.22
Lignans were reported for
the first time from pomegranate in 2009, adding to the family of phenolics
found in this species.23 Other chemical classes have also recently
been reported from pomegranate, including triterpenes,24, while the
seed oil of pomegranate was found to contain phytosterols and an unusual fatty
acid profile dominated by punicic acid, an omega-5 linolenic acid isomer with
the carbon-carbon double bonds at positions 9, 11, and 13.25 Figure
1 illustrates the more abundant polyphenolics in pomegranate (punicalagins, 1; punicalins, 2; lagerstannin C, 3; and
ellagic acid, 4).
Three different forms of
adulteration have been detected in pomegranate products in the global
marketplace: (a) addition of juices from other (lower cost) fruits to
pomegranate juice; (b) spiking of pomegranate extracts with additional EA or
polyphenols; and (c) products made mostly from unknown or unidentified source
materials, with little-to-no pomegranate constituents. EA is the principal
chemical adulterant of extracts, although its true source may be unknown (EA
can be produced by chemical synthesis and/or extraction from other natural
sources), while various sugars, organic acids, amino acids, and polyphenols can
serve as markers of adulteration by other fruit juices.
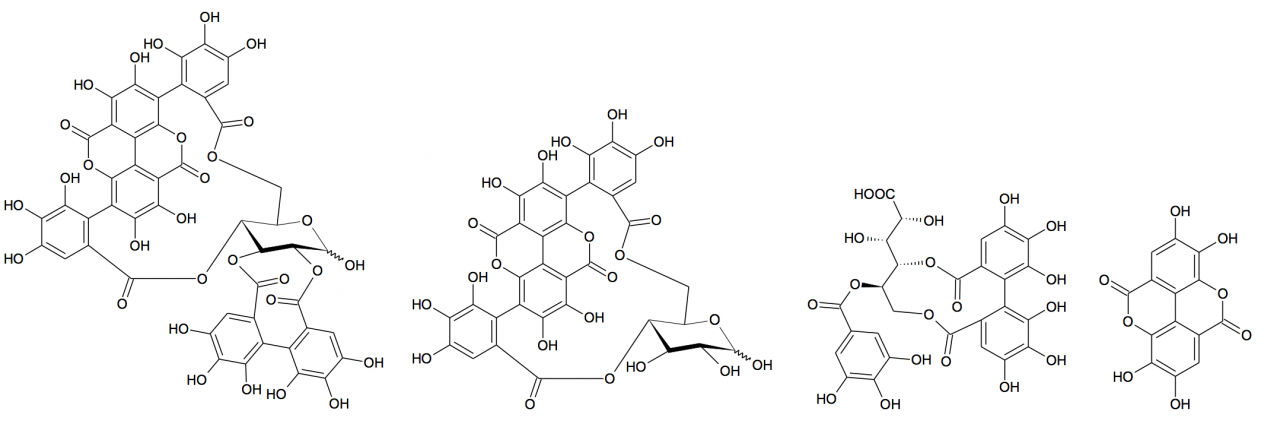
Punicalagins (1) Punicalins (2) Lagerstannin C (3) Ellagic acid (4)
Figure
1: Major polyphenols in peel and mesocarp of pomegranate fruit, according to
Fischer et al.26
8.2
Laboratory methods
Table 1 lists different
methods used to analyze commercial pomegranate juice products for adulteration
and considers the key advantages and disadvantages of each technique, while
Table 2 provides a similar comparative analysis of analytical methods for
products made from pomegranate extracts.
8.2.1
Juice
Table
1. Comparison of different analytical approaches to determine adulterants in
pomegranate juice
|
Reference
|
Sample Set
|
Method(s)
|
Analyte(s)
|
Pro
|
Contra
|
|
Zhang (2009a)27
|
45 commercial juices from 23 manufacturers
|
HPLC-UV
HPLC-RI
HPLC-UV (PDA)
ninhydrin -VIS
MS
flame photometry
|
anthocyanins, ellagitannins
sugars
organic acids – citric, isocitric, tartaric,
malic
proline
13C enrichment
of sugars
potassium
|
standard equipment in many laboratories; analytes have strong UV chromophores; some reference compounds available
reference compounds available
standard equipment in many laboratories; reference compounds available
simple, inexpensive;
reference compound available
best method to identify exogenous sugars
simple sample
preparation/procedure
|
some equipment is moderately costly
RI detectors may not be available in all
laboratories
multistep sample preparation
some equipment is moderately costly
equipment not found in all labs
|
|
Ehling (2011)28
|
6
samples freshly prepared juice; 10
commercial juice samples
|
HPLC-MS/MS
with stable isotope dilution
|
organic
acids – citric, isocitric, tartaric, malic, quinic
|
unambiguous
determination of organic acids at low mg/L levels; reference compounds available
|
equipment
is expensive
|
|
Nuncio-Jauregui (2014)29
|
pomegranate,
grape, peach juice
|
HPLC-UV
(PDA)a
HPLC-RIb
AAc
ninhydrin-VIS
HS/SPMEd-GC
|
organic
acids – citric, tartaric, malic
sugars –
glucose, sucrose, fructose
minerals
–Na,K,Ca,Mg,Fe,Zn,Cu,Mn
proline
volatile
flavor/aroma compounds
|
standard equipment in many laboratories; reference compounds available
reference compounds available
reference
materials available; simple, inexpensive
simple, inexpensive; reference compound available
standard equipment in some laboratories; reference compounds available
|
some equipment is moderately costly
RI detectors may not be available in all
laboratories
equipment not found in all labs
technique
not commonly used in supplement industry
|
|
Tezcan (2013)30
|
pomegranate,
apple juice
|
chiral
MEKC-LIFe
|
amino
acids
|
reference compounds available
|
equipment not found in all labs
|
|
Borges (2013)31
|
6 juices
marketed as pure pomegranate;
20
pomegranate/ other juice blends;
10 other
juices
|
HPLC-PDA-MS/MS
|
polyphenols
|
reference compounds available
|
equipment
is expensive
|
|
Borges (2010)32
|
4
juices, 1 wine
|
HPLC-PDA-MS-FDf
|
polyphenols
|
reference compounds available
|
equipment
is expensive
|
|
Krueger
Food Laboratories (2012)33
|
>500
juice samples
|
10
different ana-lytical methods, plus iterative statistical analysis
|
sugars,
organic acids, polyphenols
|
reference compounds available; large sample set; can detect many forms of adulteration
|
10
analytical procedures; statistical analyses required
|
|
Vardin (2008)34
|
pomegranate
and grape juice concentrate
|
FTIRg,
with chemometrics
|
fruity
esters, acids -absorptions in carbonyl range (1780-1685 cm-1)
|
non-destructive
method
inexpensive
|
may not
work for all juice adulterants
|
|
El Darra
(2017)35
|
pomegranate
and date juice concentrate
|
ATRh-FTIR
HPLC-PDA
|
fruity
esters, acids -absorptions in carbonyl range (1780-1685 cm-1)
anthocyanins
|
non-destructive
method, inexpensive
standard equipment in many laboratories;
reference compounds available
|
effective
for date juice concentrate, but may not work for all juice adulterants
moderately
expensive equipment
|
|
Gómez-Caravaca
(2013)36
|
pomegranate
juice
|
HPLC-PDA-ESI/qTOF/MS
|
13
anthocyanins and 14 other phenolic compounds
|
thorough
analysis of large number of phenolics; some reference compounds available
|
equipment
is expensive, not found in all laboratories
|
|
Brighenti
(2017)37
|
pomegranate
juice and extract
|
HPLC-DAD-ESI/MS
|
punicalagins
A and B, ellagic acid, ellagic acid hexoside, ellagic acid deoxyhexoside,
ellagic acid pentoside, cyanidin 3-O-glucoside,
cyanidin 3,5-O-diglycoside
|
method
validated, compliant with ICHi; some reference standards available
|
equipment
is moderately expensivea Photodiode array detection |
a Photodiode array detection
b Refractive index detection
c Atomic absorption spectroscopy
d Head space – solid phase microextraction
e Micellar electrokinetic chromatography-laser induced fluorescence detection
f Fluorescence detection
g Fourier transform infrared spectroscopy
h Attenuated total reflectance
i International Conference on Harmonisation (of Technical Requirements for the Registration of Pharmaceuticals)
Comments: A quick look at the contents of Table
1 illustrates that some form of HPLC-UV analysis, with or without mass
spectrometry and/or fluorescence detection, is the primary means of determining
the polyphenol content of pomegranate juice (ellagitannins, EA, and
gallotannins). This HPLC-UV approach can also highlight the presence of
polyphenolics that should not be present in authentic pomegranate juice (e.g.,
polyphenolics from grape or cranberry juice).
The challenge with
juices is determining what adulterant juices or additives (e.g., sugars,
colorants) are present. There is a variety of options available to
researchers/analytical groups, but the most useful of these appear to be
analysis of sugar content (notably glucose, sucrose, and fructose) and organic
acid content (citric, isocitric, malic, tartaric, quinic). There are cases
where mineral content or amino acid profile might be instrumental in
identifying a particular adulterant juice. 12C/13C ratios
can be used to identify cases where synthetic or exogenous sugars have been
added to a juice product.
The Krueger Food
Laboratory analysis of >500 juices/juice products using ten different
validated analytical methods, plus application of iterative statistical
analysis of the resulting data, gave very robust profiles of the chemical content
of pomegranate juice and likely or potential adulterant juices.33
This report can provide very useful guidance or insight for the selection of an
analytical strategy.
The 2017 report by
Brighenti et al.37 provides a HPLC-UV-ESI-MS2 method,
validated and compliant with ICH guidelines, for juice and extracts of peel.
The authors identified 31 peaks in the juice chromatogram as phenolic
compounds, and 51 in the chromatograms of mesocarp and exocarp extracts; oddly,
two major peaks in the juice chromatogram (monitored at 268 nm) were not
identified, even tentatively. The method described employs a fused core HPLC
column, a relatively new column technology not reported in many analyses of
botanicals, but the authors found that it gave better resolution with less
solvent consumption than either C18 or pentafluoro-phenyl phase
columns. Thus, it seems amenable to rapid adoption for use in quality assurance
and adulteration detection.
Not listed in Table 1,
but of likely interest to manufacturers and marketers of pomegranate products,
are some recent and current studies, by collaborating teams from Bruker BioSpin
GmbH and SGF International e.V., reporting the development of NMR methodologies
for the quality control of fruit juices.38,39 This work was focused
primarily on apple juice as the lead example and led, a few years later, to a
published validation study on quantitative analysis of multiple components.40
The technique requires a highly shielded 400 MHz NMR magnet with a flow
injection system, permitting a high throughput of samples. A
Bruker application
note indicates that pomegranate juice can be qualitatively and quantitatively
evaluated by this methodology.41
8.2.2
Extract
Table
2. Comparison of different analytical approaches to determine adulterants in
pomegranate extracts and extract-based products
|
Reference
|
Method(s)
|
Analytes
|
Pro
|
Contra
|
|
Zhang
(2009b)42
|
HPLC-PDA
TEACa,
GAEb and EAEc
|
punicalagins,
punicalins, ellagitannins
total
polyphenols
|
standard
equipment in most laboratories; reference standards available
|
equipment
is moderately expensive; multiple analyses involved
not
particularly useful for adulteration analyses
|
|
Madrigal-Carballo
(2009)43
|
HPLC-PDA
|
punicalagins,
punicalins, ellagitannins, gallotannins
|
standard
equipment in most laboratories; reference standards available
|
equipment
is moderately expensive
|
|
Fischer
(2011)26
|
HPLC-DAD–ESI/MSn
|
anthocyanins,
gallo-tannins, ellagitannins, gallagyl esters, hydroxy-benzoic acids,
hydroxy- cinnamic acids, dihydroflavonol
|
thorough
analysis delineating 48 phenolic constituents; some reference standards
available
|
equipment
is rather expensive, not available in all laboratories
|
|
Li
(2015)44
|
HPLC-UV
|
punicalagins A and B,
ellagic acid, gallic acid
|
standard
equipment in most laboratories; some reference standards available
|
reference
standards not available for all peaks characteristic of HPLC fingerprint
|
|
Brighenti
(2017)37
|
HPLC-DAD-ESI/MS
|
punicalagins A and B,
ellagic acid, ellagic acid hexoside, ellagic acid deoxyhexoside, ellagic acid
pentoside, cyanidin 3-O-glucoside,
cyanidin 3,5-O-diglycoside
|
method
validated, compliant with ICHd; some reference standards available
|
equipment
is moderately expensive
|
a Trolox equivalent antioxidant capacity
b Gallic acid equivalent
c Ellagic acid equivalent
d International Conference on Harmonisation
(of Technical Requirements for the Registration of
Pharmaceuticals)
Comments: Somewhat surprisingly, the Botanical
Adulterants Prevention Program retrieved only two detailed published analyses
of extract-containing products for evidence of adulteration, but the results in
both investigations were so strikingly similar that the evidence for widespread
adulteration of pomegranate supplements is considered quite strong. Zhang et
al.42 analyzed 27 commercially available pomegranate extracts and
found that only five of them contained significant amounts of the
pomegranate-specific ellagitannins (punicalagins and punicalin). They found
that 17 of the samples contained mostly EA; the remaining five extracts
contained little or no ellagitannins or EA (and little antioxidant activity).
Madrigal-Carballo et al.43 analyzed 19 commercially available
pomegranate extracts and reported that only seven produced polyphenolic
profiles indicative of pomegranate, while 13 of the extracts (including one of
the seven with a pomegranate profile) contained EA levels exceeding that
expected from arils and rind. Of the latter group, six had little or no
pomegranate ellagitannin content.
Both groups used HPLC-UV
for their analyses, since all the analytes of interest have strong UV
chromophores. Either method could be readily adapted for use in a company or
commercial analytical laboratory. Validation of any method to be employed and
the use of reference standards to verify peak identities is highly recommended.
If a mass spectrometer is available for use in the analytical method, its use
could both confirm the identity of known, anticipated compounds and help to
identify any unexpected or unknown peaks observed in the HPLC chromatograms.
The paper by Fischer et
al.26 is included in Table 2 because it is a thorough analysis of a
variety of juices and extracts by HPLC-UV-MS, providing evidence for the
presence of a total of 48 phenolic compounds across the samples analyzed. This
method has the potential to be developed and validated for use in verifying
product identity and quality, while at the same time exposing any adulteration.
The paper by Li et al.44
provides a useful HPLC-UV fingerprint of extracts of pomegranate peel, showing
consistency of 10 collections from four orchards in China. This method has the
potential to be validated for use in quality control of such extracts.
The recent report by
Brighenti et al.37 is discussed in the comments following Table 1
(Section 8.2.1); those comments are also applicable here.
Not listed in the table
is an NMR study by a group led by Larive, who examined the 1H-NMR
spectra of a mixture of punicalagins A and B at various pHs.45 The
chemical shifts (position of the NMR signals) of the aromatic protons were found
to be very sensitive to pH. The aromatic region of the NMR spectra of these
compounds is not as signal-rich as the carbohydrate region, suggesting that
this approach could conceivably be developed as a method of detection of
adulteration or decomposition of extracts and products derived from extracts.
9.
Conclusion
There is a growing body
of data indicating that pomegranate juice and extract products are frequently
adulterated. Possibly driven by supply/demand issues and/or economic
incentives, such fraudulent products deprive consumers of the health benefits
of pomegranate.
Various analytical
methods are reviewed in this guidance document, with the seemingly most broadly
applicable and fit-for-purpose of those highlighted for the benefit of readers.
Based on the available
evidence, none of the known adulterants, whether they are other fruit juices or
exogenous substances, represent an apparent safety concern to consumers.
10.
References
- Cardellina JH, II, Blumenthal M. Adulteration of pomegranate products — A
review of the evidence. HerbalGram.
2016;112:62-69.
-
Graham SA, Hall J, Sytsma K, Shi S-H. Phylogenetic analysis of the
Lythraceae based on four gene regions and morphology. Int J Plant Sci. 2005;166(6):995-1017.
-
Morton JF. Pomegranate, Punica granatum L. In: Morton JF. Fruits of Warm
Climates. 1987; 352–355. Purdue New Crops Profile website. Available at: https://www.hort.purdue.edu/newcrop/morton/pomegranate.html. Accessed May 23, 2017.
-
Bhandari PR. Pomegranate (Punica granatum L).
Ancient seeds for modern cure? Review of potential therapeutic applications. Int J Nutr Pharmacol Neurol Dis. 2012;2:171-184.
-
Ruis
AR. Pomegranate and the mediation of balance in early medicine. Gastronomica. 2015;15:22-33.
-
Miguel MG, Neves MA, Antunus MD. Pomegranate (Punica granatum L.): A medicinal plant
with myriad biological properties – A short review. J Med Plants Res. 2010;4:2836-2847.
- Jasuja ND, Saxena R, Chandra S, Sharma R. Pharmacological
characterization and beneficial uses of Punica
granatum. Asian J Plant Sci. 2012;6:251-267.
- Katz SR, Newman RA, Lansky EP. Punica granatum: Heuristic treatment for diabetes mellitus. J
Med Food. 2007;10:213-217.
- Jurenka J. Therapeutic applications of pomegranate (Punica granatum L.): A review. Alt Med Rev. 2008;13:128-144.
- Yuan T, Ma H, Liu W, Niesen DB, Shah N, Crews R, Rose KN, Vattem DA, Seeram NP. Pomegranate’s neuroprotective effects
against Alzheimer’s disease are mediated by
urolithins, its ellagitannin-gut microbial derived metabolites.
ACS Chem Neurosci. 2016;7:26−33.
- BenSaad LA, Kim KH, Quah CC, Kim WR,
Shahimi M. Anti-inflammatory potential of ellagic acid, gallic acid and
punicalagin A&B isolated from Punica
granatum. BMC Compl Alt Med.
2017;17:47-56.
- Liu C, Cai D, Zhang L, Tang W, Yan R, Guo
H, Chen X. Identification of hydrolysable tannins (punicalagin, punicalin and
geraniin) as novel inhibitors of hepatitis B virus circularly closed DNA. Antiviral Res. 2016;134:97-107.
- WHO Monographs
on Selected Medicinal Plants. Volume 4. World Health Organization, Geneva, 2009; 118.
- The Ayurvedic
Pharmacopoeia of India. Part I, Volume II. New Delhi, India: Ministry of Health and
Family Welfare, Government of India. 1999: 32.
- Gohil KM, Prajapati PK, Harisha CR. Detailed micromorphological and
pharmacognostic evaluation of Dadima fruit (Punica
granatum). Am J PharmTech Res.
2015;5:237-244.
- Marieschi
M, Torelli A, Beghé D, Bruni R. Authentication of Punica granatum L.: Development of SCAR markers for the detection
of 10 fruits potentially used in economically motivated adulteration. Food Chem. 2016;202:438-444.
- Zhang YP, Tan HH, Cao SY, Wang XC, Yang G,
Fang JG.
A novel strategy for identification of 47 pomegranate (Punica granatum) cultivars using RAPD
markers. Genetics Mol Res. 2012;11:3032-3041.
- Sarkhosh A, Zamani Z, Fatahi R, Ebadi A. RAPD markers reveal
polymorphism among some Iranian pomegranate (Punica granatum L.) genotypes. Sci
Horticult. 2006;111:24-29.
- Masoud S, Saneghi A, Shahreiyari ZH, Noormohammadi Z, Farahanei F, Tabatabaei-Ardakanei SZ. RAPD and
cytogenetic study of some pomegranate (Punica
granatum L.) cultivars. Caryologia 2008;61:68-73.
- Hasnaoui N, Messaoud M, Jemni C, Mokhtar T. Molecular
polymorphisms in Tunisian pomegranate (Punica
granatum L.) as revealed by RAPD fingerprints. Diversity 2010;2:107-114.
- National
Center for Biotechnology Information. Random Amplified Polymorphic DNA (RAPD). Available at: https://www.ncbi.nlm.nih.gov/probe/docs/techrapd/. Accessed January 25, 2018.
- Ambigaipalan
P, de Camargo AC, Shahidi F. Phenolic compounds of pomegranate byproducts
(outer skin, mesocarp, divider membrane) and their antioxidant activities. J Agric Food Chem. 2016;64:6584-6604.
- Bonzanini F, Bruni R, Palla G, Serlataite N, Caligiani A.
Identification and distribution of lignans in Punica granatum L. fruit endocarp, pulp, seeds, wood knots and
commercial juices by GC-MS. Food Chem. 2009;117:745-749.
- Jiang
HZ, Ma QY, Fan HJ, Liang WJ, Huang SZ, Dai HF, Wang PC, Ma XF, Zhao YX. Fatty acid synthase inhibitors isolated from Punica granatum L. J Braz Chem Soc. 2012;23:889-893.
- Kaufman M, Wiesman Z. Pomegranate oil analysis with
emphasis on MALDI-TOF/MS triacylglycerol fingerprinting. J Agric Food Chem. 2007;55:10405–10413.
- Fischer UA, Carle R, Kammerer DR. Identification and
quantification of phenolic compounds from pomegranate (Punica granatum L.) peel, mesocarp, aril and differently produced
juices by HPLC-DAD–ESI/MSn.
Food Chem. 2011;127:807–821.
- Zhang
Y, Krueger D, Durst R, Lee R. Wang D, Seeram N, Heber D. International
multidimensional authenticity specification (IMAS) algorithm for detection of
commercial pomegranate juice adulteration. J
Agric Food Chem. 2009;57:2550-2557.
- Ehling S, Cole S. Analysis of organic acids in fruit
juices by liquid chromatography-mass spectrometry: An enhanced tool for
authenticity testing. J Agric Food Chem.
2011;59:2229–2234.
- Nuncio-Jauregui N,
Calin-Sanchez A, Hernandez F, Carbonell-Barrachina AA. Pomegranate juice adulteration by addition of grape or peach juices. J
Sci Food Agr. 2014;94:646-655.
- Tezcan F, Uzaşçi S, Uyar
G, Oztekin N, Erim FB. Determination of amino acids in pomegranate juices and
fingerprint for adulteration with apple juices. Food Chem. 2013;141:1187-1191.
- Borges G, Mullen W, Crozier A. Comparison of the
polyphenolic composition and antioxidant activity of European commercial fruit
juices. Food Funct. 2010;1:73–83.
- Borges
G, Crozier A. HPLC-PDA-MS fingerprinting to assess the authenticity of
pomegranate beverages. Food Chem. 2012;135:1863-1867.
- Krueger
DA. Composition of pomegranate juice. J
AOAC Int. 2012; 95:163-168. See
also: Krueger Food Laboratories, Fruit
juice authenticity analysis. Available at: http://www.kfl.com/pom.html, accessed 12 September 2017.
- Vardin H, Yay A, Ozen B,
Mauer L. Authentication of pomegranate juice concentrate using FTIR
spectroscopy and chemometrics. Food Chem.
2008;108:742-748.
- El
Darra N, Rajha HA, Saleh FN, Al-Oweini R, Maroun R, Louka N. Food fraud
detection in commercial pomegranate molasses syrups by UV–VIS spectroscopy,
ATR-FTIR spectroscopy and HPLC methods. Food
Cont. 2017;78:132-137.
- Gómez-Caravaca
AM, Verardo V, Toselli M, Segura-Carretero A, Fernández-Gutiérrez A, Caboni
MF. Determination of the
major phenolic compounds in pomegranate juices by HPLC−DAD−ESI-MS. J Agric Food Chem. 2013;61:5328-5337.
- Brighenti V, Groothuis SF, Prencipe FP, Amir R, Benvenuti
S, Pellati F. Metabolite fingerprinting of Punica granatum L. (pomegranate) polyphenols
by means of high-performance liquid chromatography with diode array and
electrospray ionization-mass spectrometry detection. J Chromatogr A. 2017;1480:20-31.
- Spraul M, Schütz B, Humpfer E, Mörtter M, Schäfer H,
Koswig S, Rinke P. Mixture
analysis by NMR as applied to fruit juice quality control. Magn Res Chem. 2009;47:S130-S137.
- Spraul M, Schütz B, Rinke P, Koswig S, Humpfer E, Schäfer
H, Mörtter M, Fang F, Minoja A. NMR-based
multi parametric quality control of fruit juices: SGF profiling. Nutrients. 2009;1:148-155.
- Monakhova YB, Schütz B, Schäfer H, Spraul M, Kuballa T,
Hahn H, Lachenmeier DW. Validation studies for multicomponent quantitative NMR
analysis: the example of apple fruit juice. Accred
Qual Assur. 2014;19:17-29.
- NMR (Nuclear Magnetic Resonance) screening of juices,
pulps and purees now significantly enhanced through increased quantification
parameters and wider coverage, providing reliable targeted and non-targeted multi-marker
analysis. Bruker website. Available at: https://www.bruker.com/news-records/single-view/article/bruker-announces-next-generation-juicescreener-30.html.
Accessed March 14, 2018.
- Zhang Y, Wang D, Lee RP, Henning SM, Heber D. Absence of
pomegranate ellagitannins in the majority of commercial pomegranate extracts:
implications for standardization and quality control. J Agric Food Chem. 2009; 57:7395-7400.
- Madrigal-Carballo S, Rodriguez G, Krueger CG, Dreher M,
Reed JD. Pomegranate (Punica granatum)
supplements: Authenticity, antioxidant and polyphenol composition. J Funct Food. 2009;324-329.
- Li J, He X, Li, Zhao W, Liu L, Kong X. Chemical
fingerprint and quantitative analysis for quality control of polyphenols
extracted from pomegranate peel by HPLC. Food
Chem. 2015;176:7-11.
- Kraszni M, Marosi A, Larive CK. NMR assignments and the
acid–base characterization of the pomegranate ellagitannin punicalagin in the
acidic pH-range. Anal Bioanal Chem. 2013;405:5807-5816.