 Click here for PDF
Click here for PDF
Saw Palmetto Extract Laboratory Guidance Document
By Stefan
Gafner, PhD*
American
Botanical Council, Austin, TX 78723
*Correspondence: email
Keywords: Adulteration, animal fatty
acids, canola oil, coconut oil, palm oil, saw palmetto, Serenoa repens, sunflower oil, vegetable oil
Citation (JAMA)
style: Gafner S. Saw palmetto extract laboratory guidance document. Austin, TX:
ABC-AHP-NCNPR Botanical Adulterants Prevention Program. 2019.
CONTENTS
1. Purpose
2. Scope
3. Common and Scientific Names
3.1 Common name
3.2 Other common names for saw palmetto
3.3 Latin binomial
3.4 Synonyms
3.5 Botanical family
4. Botanical Description and Geographical Range
5. Adulterants and Confounding Materials
Table 1. Scientific Names, Family, and Common Names of Plants Used as Sources of Vegetable Oils Known as Saw Palmetto Fruit Extract Adulterants
6. Identification and Distinction using Macroanatomical Characteristics
7. Identification and Distinction using Microanatomical Characteristics
8. Organoleptic Identification
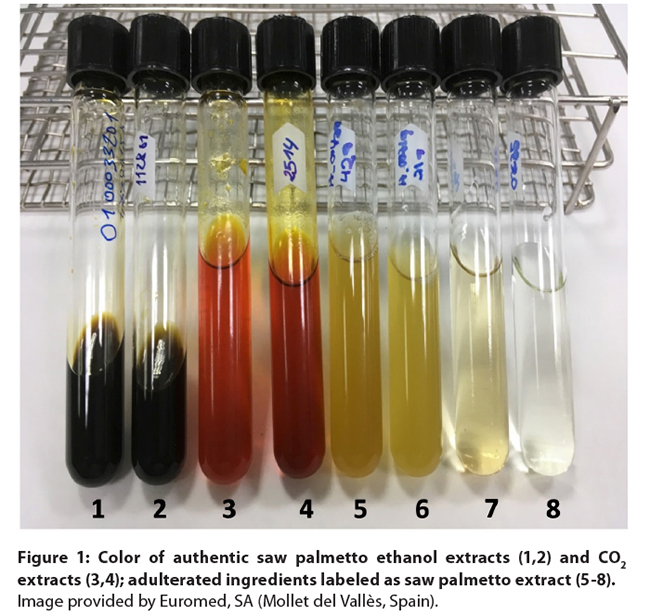
Figure 1: Color of authentic saw palmetto ethanol extracts (1,2)
and CO2 extracts (3,4); adulterated ingredients labeled as saw
palmetto extract (5-8). Image
provided by Euromed, SA (Mollet del Vallès, Spain).
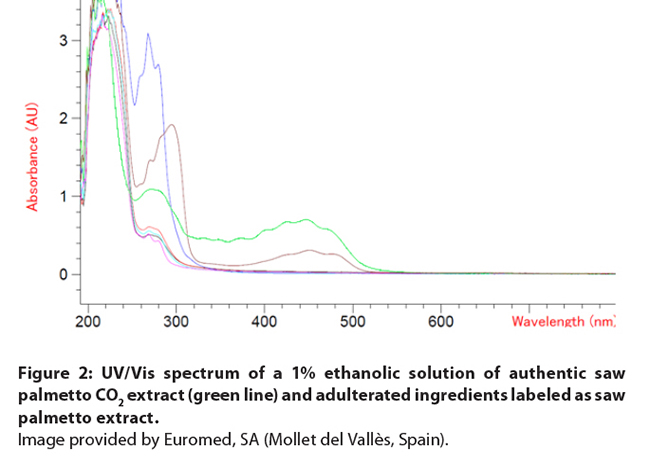
Figure
2: UV/Vis spectrum of a 1% ethanolic solution of authentic saw palmetto CO2
extract (green line) and adulterated ingredients labeled as saw palmetto
extract. Image provided by Euromed, SA (Mollet del Vallès,
Spain).
9. Genetic Identification and Distinction
10. Physicochemical Tests
11. Chemical Identification and Distinction
11.1 Chemistry of Serenoa repens and potential adulterants
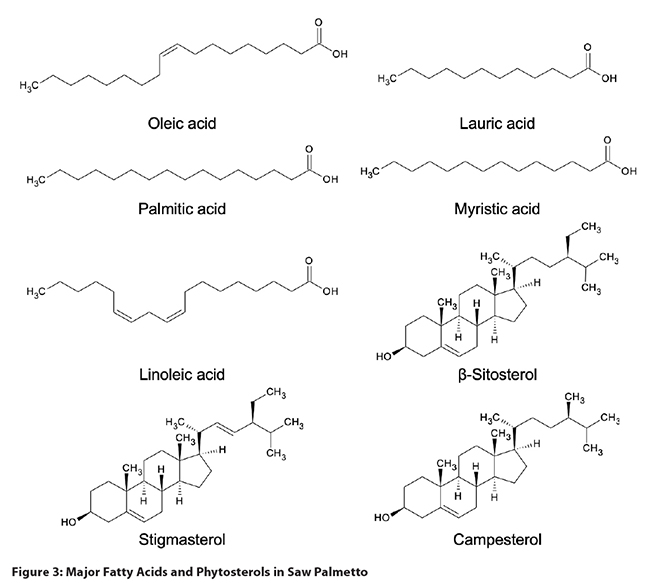
Figure 3: Major Fatty Acids and Phytosterols in Saw Palmetto
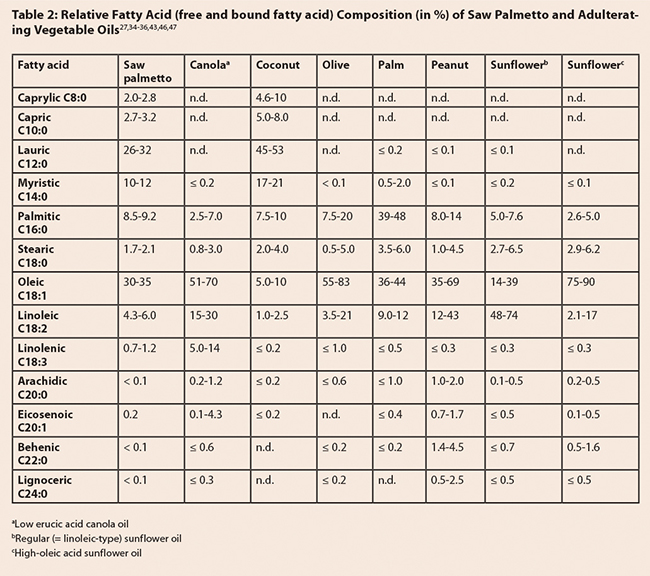
Table 2: Relative Fatty Acid (free and bound fatty acid) Composition (in
%) of Saw Palmetto and Adulterating Vegetable Oils
11.2
Laboratory methods
11.2.1 High-Performance Thin-Layer Chromatography
11.2.2 Infrared spectroscopy
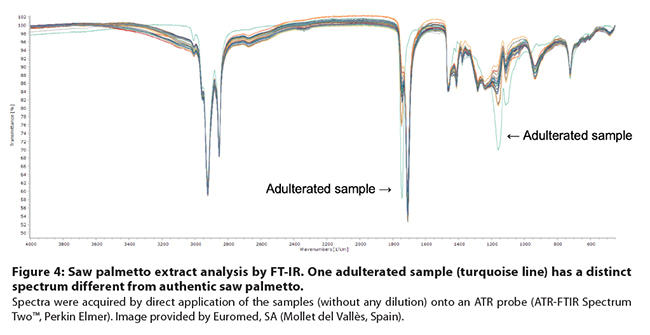
Figure
4: Saw palmetto extract analysis by FT-IR. One adulterated sample (turquoise line)
has a distinct spectrum different from authentic saw palmetto. Spectra
were acquired by direct application of the samples (without any dilution) onto
an ATR probe (ATR-FTIR Spectrum Two™, Perkin Elmer). Image provided by
Euromed, SA (Mollet del Vallès, Spain).
11.2.3
High-performance liquid chromatography
11.2.4 Gas chromatography
Table 3: Comparison among GC Methods to Determine Fatty Acids in Saw Palmetto
Extracts.
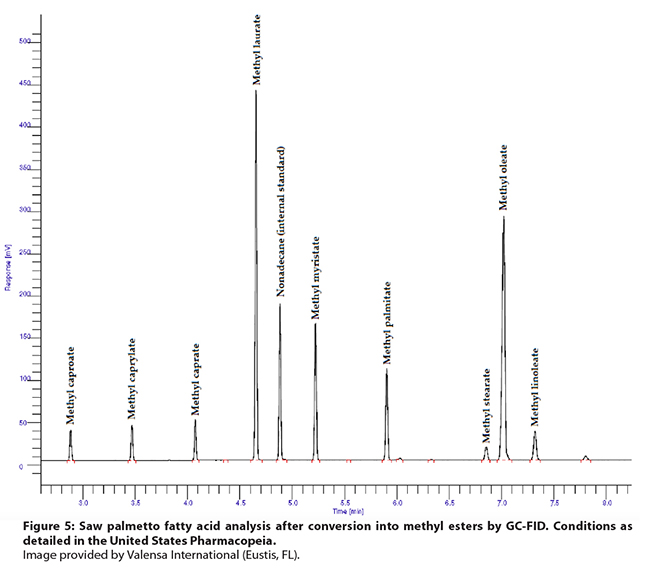
Figure 5: Saw palmetto fatty acid analysis after conversion into methyl
esters by GC-FID. Conditions as detailed in the United States Pharmacopeia. Image provided by Valensa International (Eustis, FL).
11.2.5 Nuclear magnetic resonance
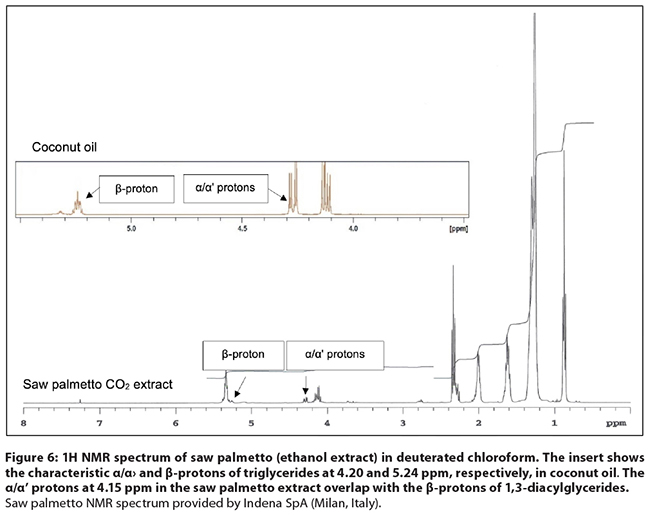
Figure
6: 1H NMR spectrum of saw palmetto (ethanol extract) in deuterated chloroform.
The insert shows the characteristic α/α' and β-protons of
triglycerides at 4.20 and 5.24 ppm, respectively, in coconut oil. The α/α' protons at 4.15 ppm in the saw
palmetto extract overlap with the β-protons of 1,3-diacylglycerides. Saw
palmetto NMR spectrum provided by Indena SpA (Milan, Italy).
11.2.6 Stable isotope ratio
Table 4. Comparison among the Different Chemical
Methods to Authenticate Saw Palmetto Extract
12. Conclusion
13. References
1.
Purpose
There is documented evidence
of the adulteration of saw palmetto fruit extracts with a number of vegetable
oils, such as canola (Brassica napus ssp. napus, Brassicaceae), coconut (Cocos
nucifera, Arecaceae), olive (Olea
europaea, Oleaceae), palm (Elaeis
guineensis, Arecaceae), peanut (Arachis
hypogaea, Fabaceae), and sunflower (Helianthus
annuus, Asteraceae) oils. The partial or complete substitution of saw
palmetto fruit extracts with mixtures of fatty acids of animal origin was first
documented in 2018,1 and seems
particularly common in materials sold as saw palmetto originating from China. This
Laboratory Guidance Document (LGD) presents a review of the various analytical
technologies used to differentiate between authentic saw palmetto extracts and ingredients
containing adulterating materials. This document can be used in conjunction
with the Saw Palmetto Botanical Adulterants Bulletin, rev. 3, published by the
ABC-AHP-NCNPR Botanical Adulterants Prevention Program in 2018.2
2.
Scope
Various analytical methods are
reviewed here with the specific purpose of identifying their strengths and
limitations in differentiating saw palmetto fruit extracts from potentially
adulterating materials. Less emphasis is given to the authentication of whole,
cut, or powdered saw palmetto fruit and distinguishing it from potential
confounding materials, e.g., the Everglades palm (Acoelorrhaphe wrightii, Arecaceae), by macroscopic, microscopic or
genetic analysis. Analysts can use this review to guide their selection of appropriate
analytical authentication techniques. The suggestion of a specific analytical method
for testing saw palmetto materials in their particular matrix in this LGD does not reduce or remove the
responsibility of laboratory personnel to demonstrate adequate method
performance in their own laboratories using accepted protocols. Such protocols
are outlined in the United States Food and Drug Administration’s Good
Manufacturing Practices (GMPs) rule (21 CFR Part 111) and those published by
AOAC International, International Organization for Standardization (ISO), World
Health Organization (WHO), and International Conference on Harmonisation (ICH),
and national pharmacopeial bodies, as may be applicable, depending on the
regulatory requirements of the country in which the saw palmetto extract is
being offered for sale, re-sale, and/or processing into finished consumer
products.
3.
Common and Scientific Names
3.1
Common name: Saw palmetto
3.2
Other common names for saw palmetto
English: Scrub-palmetto, sabal palm, saw palmetto berry
Chinese: Ju zonglu (锯棕榈)
French: Sabal, palmier nain, palmier scie
German: Sabal, Sägepalme, Zwergpalme
Italian: Palma nana, cavolo di palma
Russian: Сереноя ползучая (Serenoa repens),
Сабаль пильчатый (Sabal serrulata), карликовая пальма (karlikovaya palma, “dwarf
palm”), пальма cереноа, co пальметто
Spanish: Sabal, palma enana americana3,4
Swedish: sågpalmetto
3.3 Latin binomial: Serenoa repens (W. Bartram) Small
3.4 Synonyms: Chamaerops serrulata
Michx., Corypha obliqua W. Bartram, Corypha repens W. Bartram, Diglossophyllum serrulatum
(Michx.) H. Wendl. ex Drude, Sabal serrulata
(Michx.) Nutt. Ex Schult. & Schult. f., Serenoa
serrulata (Michx.) G. Nicholson5
3.5
Botanical family: Arecaceae
4.
Botanical Description and Geographical Range
Saw palmetto grows as a
small shrub, occasionally a small tree with creeping, horizontal, branched
stems, usually to a height of 2-7 feet (0.6-2.1 m), although it may reach up to
25 feet (7.5 m). The stem systems run parallel to the soil surface, eventually
branching beneath the substrate to form rhizomes. Saw palmetto leaves are
fan-shaped, evergreen and about 3 feet (1 m) wide. The margins of the petioles
are lined with sharp spines that have given saw palmetto its common name. The
flowers are cream-colored and fragrant, with three petals at the end of stalked
panicles that grow from the leaf axils. The fruit is a drupe, green or yellow
at immature stages, and black when ripe (between August and October), resembling
black olives in size and shape.6,7 The plant is endemic
to the southeastern United States, growing from the coastal plains of Louisiana
across the Florida peninsula and up to South Carolina.6
5.
Adulterants and Confounding Materials
The few reports of
adulteration of saw palmetto berries with berries from related species, i.e., dwarf
palmetto (Sabal minor),13,14 queen palm (Syagrus romanzoffiana),14 and Everglades palm
(Acoelorrhaphe wrightii)15 of the palm family (Arecaceae)
seem to suggest that such adulteration is rare. Fruits of dwarf palmetto (6-12
mm) and the Everglades palm (10-15 mm) are smaller and spherical compared to
saw palmetto fruit, which is oval-shaped, of ca. 15 mm width and 12-25 mm
length.4,13,14,16 Compared to saw palmetto, the fruit of
queen palm is larger (20-25 mm) and heavier.14
6.
Identification and Distinction using Macroanatomical Characteristics
Botanical descriptions of saw palmetto fruit have been
published in a number of pharmacopeial monographs and books.4,17-19 Criteria to distinguish saw palmetto
fruits from other fruits in their whole form have been published by many
authors.16,20,21 The macroscopic assessment is the
method of choice to distinguish unripe (green) from semi-mature or mature
(black) berries and is sufficient for identifying saw palmetto to species. For
obvious reasons, macroscopic identification is not applicable to saw palmetto
extracts.
7.
Identification and Distinction using Microanatomical Characteristics
Microscopic
descriptions of saw palmetto are found in the pharmacopeias of Europe and the
United States, and the American Herbal Pharmacopoeia’s textbook on microscopic
characterization of botanical medicines.17,18,22 Details of the fruit anatomy of saw palmetto,
Everglades palm, and dwarf palmetto have been published by Zona;21 however, no clear differentiation criteria for the
fruits of these palms using botanical microscopy were provided.
8.
Organoleptic Identification
Saw palmetto berries are
initially sweet, then pungent, acrid, and saponaceous. The aroma is strongly
aromatic and foul, reminiscent of foul-smelling socks. Saw palmetto extracts
also have a distinct aromatic and foul odor. Color can provide an indication to
detect adulterated ingredients (Figures 1 and 2). Ethanol, hexane, and high
pressure CO2 extracts of saw palmetto from ripe berries typically
have a dark green-brown color due to the extraction of chlorophyll with these
solvents. Low pressure CO2 extracts have a yellow to orange-brown
color. While organoleptic evaluation does not provide sufficient safeguard
against adulteration, the extract color and the very characteristic odor are
helpful in assessing the authenticity of a saw palmetto extract.
9.
Genetic Identification and Distinction
Several authors have looked into differences among
nucleotide sequences of various gene regions for saw palmetto and closely
related palm species, including dwarf palm and Everglades palm. In most cases,
the purpose was to determine phylogenetic relationships.23-26 Nucleotide sequences of the chloroplast
(atpB, matK, ndhF, rbcL, rps16 intron, trnD-trnT, trnL-trnF, trnQ-rps16) and nucleus (18S,
ITS, ms, prk, rpb2) were used to establish the
relationship among members of the palm family.25,26 Genome skimming was applied to assemble
the entire chloroplast nucleotide sequence in leaf samples of 29 palm species,
including saw palmetto and the Everglades palm.23 Genetic data on commercial saw palmetto
products are less abundant. Nevertheless, Little and Jeanson15 investigated the authenticity of 37 commercial saw
palmetto products containing dried, cut and sifted plant material using
mini-barcodes from the matK and the rbcL regions. Amplifiable DNA was obtained
for 34 samples (92%), with 29 (85%) samples containing saw palmetto. In three
samples, only the matK mini-barcode sequence
was obtained, which was insufficient to distinguish between saw palmetto and
its closest relative, the Everglades palm. Two products were made from fruit of
other palm trees; one was made solely from Everglades palm fruit, while for the
other, the exact species could not be determined.15
Comments: Based on the report described above,15 the use of DNA mini-barcoding is a suitable means for authentication of crude saw palmetto fruit materials.27 However, the use of genetic techniques to determine the authenticity of saw palmetto extracts is not appropriate because fatty oils are generally devoid of DNA of appropriate
quality to permit reliable identification.28
10.
Physicochemical Tests
The European Pharmacopoeia (Ph.
Eur.) monograph for saw palmetto extract includes specifications for the relative
density, refractive index, acid value, iodine value, and peroxide value in saw
palmetto extracts.29 Among these tests,
the determination of the acid value is the most important for the establishment
of saw palmetto extract authenticity. Since saw palmetto extracts contain a
large concentration of free fatty acids, the acid value is much higher for saw
palmetto than for other vegetable oils.27,30 While this simple test is helpful in
detecting saw palmetto adulteration, the acid value assay must be used in
combination with an appropriate chemical test to rule out adulteration with
some of the materials mentioned in section 5.
The peroxide value is a
function of fatty acid oxidation (rancidity), and therefore does not provide
any helpful information for authenticity determination. The relative density
and refractive index of saw palmetto and common vegetable oils do not differ
substantially, and thus these analytical measurements are also not useful in
establishing adulteration.27 With the exception
of palm oil, the iodine value of most vegetable oils is either above or below
the value of saw palmetto extract specified by the Ph. Eur.27 However, a material
that is compliant with the iodine value specifications of saw palmetto oil in Ph.
Eur. is easily obtained by using a suitable mixture of vegetable oils.
11.
Chemical Identification and Distinction
Chemical authentication of
saw palmetto extracts has long been dominated by gas chromatographic (GC)
methods, either analyzing the fatty acids directly, or after conversion into
fatty acid esters. In addition to GC, thin-layer chromatography (TLC) is an
integral part for identity testing in pharmacopeial monographs. Other methods
of chemical authentication are less common, although a number of additional
techniques have been investigated and are discussed below. Distinction based on
the phytochemical profile requires detailed knowledge of the constituents of saw
palmetto and its likely adulterants. Some of the important saw palmetto constituents
and their significance in authentication are discussed below. The overview of potential
adulterants is based on published literature. The chemical composition of
vegetable oils is dependent on the refining processes. Refining usually leads
to a substantial loss of phytosterols, tocopherols, and phospholipids. However,
there are numerous other vegetable oils that may be used as undeclared
substituents of saw palmetto extracts.
11.1
Chemistry of Serenoa repens and potential
adulterants
Serenoa repens: Ripe
saw palmetto fruit contains 15-20% lipids, primarily free fatty acids, fatty acid
esters, triglycerides, and sterols (Figure 3). The fruit is also rich in acidic
polysaccharides. Additional compounds include phenolic acids such as 4,5-di-O-caffeoylshikimic acid, 4,5-di-O-caffeoylquinic acid, and gallic acid,
as well as flavonoids (rutin, isoquercitrin, and astragalin), and carotenoids.4,13,31 Based on Peng et al., immature berries
contain lower concentrations of fatty acids than mature berries, and approximately
the same amounts of lauric and oleic acids (compared to mature berries, where
oleic acid concentrations are higher than those of lauric acid).14 The main compounds
in saw palmetto oil are free fatty acids (70-95%), followed by glycerides
(mono-, di-, and triglycerides [5-6%]), fatty acid methyl esters (ca. 2%), phytosterols
(0.20-0.50%) and fatty alcohols (0.15-0.35%).29,32,33 Ethanol extracts are distinct from
hexane and CO2 extracts by the relatively high concentration of
phosphorylated glycerides. Hexane extracts contained a higher proportion of
free fatty acids, but lower amounts of mono-, di-, and triglycerides.32 The fatty acid
composition (numbers in parentheses refer to the percentage of fatty acid
compared to total [free and bound] fatty acids in a CO2 extract) is dominated
by oleic (30-35%) and lauric (26-32%) acids, followed by myristic (10-12%),
palmitic (8.5-9.2%), and linoleic (4.3-6.0%) acids.34-36 Marti et al. reported the presence of
hydroxylated fatty acids (12-hydroxy-5,8,10,14-eicosatetraenoic acid,
10,11-dihydro-12-oxo-15-phytoenoic acid, corchorifatty acid F) in commercial
saw palmetto extracts.32 The fatty acid
composition can be used to distinguish saw palmetto extracts from most
vegetable oils (Table 2), although some oil blends are designed to mimic the authentic
saw palmetto fingerprint in order to make the detection of adulteration more
difficult. The main phytosterols are β-sitosterol (68-72% of total sterols),
campesterol (20-23%), and stigmasterol (8-9%).36,37 Δ5-avenasterol, Δ7-avenasterol,
clerosterol, 24-methylenecholesterol, and Δ7-stigmasterol have
been reported present in minor amounts.1,38 Fatty alcohols include octacosanol,
hexacosanol, tetracosanol, and triacontanol.33,39
Arachis hypogaea oil: Peanut oil contains
approximately 96% triglycerides, with oleic, linoleic, and palmitic acids as
the main fatty acids (see Table 2).40 In addition, peanut
oil contains approximately 0.50% phospholipids and 0.30% phytosterols
(β-sitosterol, campesterol, stigmasterol, and Δ5-avenasterol).41
Brassica napus oil: Canola oil contains
94.9-99.1% triglycerides, with oleic acid making up over 60% of the total fatty
acids. Other major fatty acids in canola oil are palmitic and linoleic acids
(Table 2).40,42 Commercial canola
oil used in products on the market is usually low in erucic acid (< 2%). There
are some high erucic acid oils, which are most often referred to as rapeseed
oils (although the name sometimes is used interchangeably with canola oil),
with erucic acid content over 50% of total fatty acids. Unique to canola oil is
the presence of sulfur-containing fatty acids (epithiostearic acids). The
concentration of sterols varies between 0.70-1.00%, mainly represented by
β-sitosterol, campesterol, and brassicasterol. Since brassicasterol is unique
to Brassica oils, it can be used as a
marker compound to detect adulteration with canola oil.41 The content of
phospholipids is between 0.10-2.50%, depending on processing, with water- or
acid-degummed oils having lower contents (0.10-0.60%).
Cocos nucifera oil: The triglyceride content in
coconut oil is reported to be approximately 97%. One of the features of coconut
oil is that it contains mainly saturated fatty acids. Lauric acid (45.1-53.2%
of total fatty acids) represents the most abundant fatty acid, followed by
myristic, palmitic, and oleic acids (Table 2).27,43 The sterol content
is 0.04-0.12%, dominated by β-sitosterol (32-51% of total sterols), Δ5-avenasterol
(20-41%), and stigmasterol (11-16%).27,41
Elaeis guineensis oil: Fatty acids in palm oil
exist mainly as triglycerides (92-96%) and of diglycerides (4-7%). The latter are
represented primarily by palmitoyloleoyl-,
dioleoyl- and dipalmitoyl-glycerols, and can be used to differentiate palm oil
from other vegetable oils. In commercial palm oils, the 1,3-diacylglycerols are
more abundant than the corresponding 1,2-diacylglycerols.41,44 Palm oil has approximately
equal amounts of unsaturated and saturated fatty acids. The fatty acid
composition is dominated by palmitic and oleic acids, with lesser amounts of
linoleic and stearic acids (Table 2).27,41 Minor components of
palm oil include 0.03-0.07% sterols (consisting of 55-67% β-sitosterol
and 19-28% campesterol), 0.10-0.30% glycolipids, and 0.02-0.10% tocopherols.
The red color of crude palm oil is due to the presence of carotenoids
(0.05-0.07%).27,41
Helianthus annuus oil: As with other vegetable
oils, sunflower oil is composed mainly of triglycerides (up to 97%). Using
selective breeding techniques, sunflower seeds with different fatty acid
compositions have been developed, with regular, high-oleic and mid-oleic types
being the most common. The fatty acid compositions of regular and high-oleic
acid sunflower oils are presented in Table 2. The mid-oleic acid type is the
most popular sunflower oil in the US retail market, with approximately 55-75%
oleic, 15-35% linoleic, 5% stearic, and 4% palmitic acids.41,43,45 The sterol content in sunflower seed
oil is between 0.17-0.52%. Of this, 42-70% is represented by β-sitosterol,
5-13% by stigmasterol and campesterol, respectively, and up to 9% Δ7-stigmastenol.
The latter has not been reported in saw palmetto and could be used as a marker
for adulteration with sunflower oil. While Δ7-stigmastenol can be
eliminated by heat treatment or bleaching, these treatments result in a
conversion of Δ7-stigmastenol into the corresponding (Δ8,14)- and
Δ14-sterols.
Depending on the extent of refinement, sunflower oil has one of the highest
concentrations of α-tocopherol (0.04-0.11%) of all vegetable oils, and contains
0.72-0.86% phospholipids (the latter are not found in refined sunflower oil).27
Olea europaea oil: Olive oil is composed mainly of triglycerides (ca. 99%), with oleic,
linoleic, and palmitic acids as the most abundant fatty acids (Table 2).
46-48
The sterol content is between 0.1-0.2%, composed of a mainly β-sitosterol
(75-90%), Δ
5-avenasterol
(5-20%), and campesterol (up to 4%). Numerous unusual phytosterols (e.g., Δ
7-avenasterol, Δ
7-stigmastenol)
are present at low concentrations.
48 The content of squalene, a phytosterol precursor, is between
0.02-0.75%. Olive oil also contains pigments such as pheophytin, α- and
β-carotene, and lutein.
48 While the phenolics content is low, some of these molecules are rather
unique and can be used as specific markers for olive oil. Of particular
interest are the secoiridoids, with oleuropein (≤ 0.035%), oleocanthal
(0.004-0.021%), and oleacein (0.002-0.48%) as the most abundant.
49,50
11.2
Laboratory methods
Table
4, which appears at the end of this section, provides a summary comparison of different
methods of analysis of saw palmetto oil.
11.2.1
High-Performance Thin-Layer Chromatography
Methods from the following sources were evaluated in this
review: Ph. Eur. 9.1,17,29 the HPTLC
Association,51 and Halkina and Sherma.52
Comments: The
HPTLC conditions in both documents include a relatively non-polar mobile phase
combination with 1% acetic acid to prevent peak tailing on silica gel plates. The
detection is carried out with anisaldehyde17,29 or phosphomolybdic
acid52 reagent. The conditions employed by Halkina
and Sherma provide a rough separation into compound categories: triglycerides,
free fatty acids, and phytosterols.52 Identification of
target analytes in the Ph. Eur. is not provided, although Melzig et al.4 suggest that the
method identifies the presence of lauric acid, oleic acid, and β-sitosterol,
which is not sufficient for the detection of adulteration. Images of
representative saw palmetto extract HPTLC fingerprints using the Ph. Eur. conditions
can be viewed on the website of the HPTLC Association,51 since the conditions
are the same as in the Ph. Eur. Based on the paper by Halkina and Sherma, total
substitution with vegetable oils can be determined by the larger concentrations
of triglycerides. However, HPTLC is not the method of choice to detect
admixture of vegetable oils, or the presence of designer blends with a similar
fatty acid profile to saw palmetto extract.
11.2.2
Infrared spectroscopy
Two methods, Hanson et al.53 and Villar and Mulà,54 to detect saw
palmetto extract adulteration using infrared spectroscopy were identified for
this review.
Comments: Hanson
et al. evaluated the authenticity of 16 retail samples of saw palmetto products
by infrared (IR) spectroscopy and subsequent chemometric analysis using
principal component analysis (PCA). The addition of vegetable oils was readily
detected due to the higher content of triglycerides.53 Similarly, Villar
and Mulà presented the results of an analysis of 28 saw palmetto samples using
FT-IR (Figure 4) followed by PCA. The method clearly distinguished between
authentic and adulterated extracts.53,54 Based on these investigations,
IR spectroscopy combined with appropriate statistical methods may be suitable
for detection of palmetto extract adulteration with vegetable oils. However, adulterants
with low triglyceride content may be missed.
11.2.3
High-performance liquid chromatography
Methods described in the following articles were
evaluated in this review: Bedner et al.,55 Fibigr et al.,56 Marti et al.,32 and Al-Achi et al.57
Comments:
High-performance liquid chromatography (HPLC) is rarely used for the analysis
of saw palmetto due to the challenges in resolving the analytes of interest,
and their lack of a chromophore.
Phytosterol
analysis: Three HPLC methods evaluated as part of this laboratory
guidance document, were developed for the analysis of phytosterols using either
a RP-18 or a phenyl column, with mass spectrometric (MS) detection. Bedner et
al. developed two isocratic HPLC methods (comparing RP-18 and phenyl columns) for
the separation of campesterol, cycloartenol, lupenone, lupeol, β-sitosterol, and
stigmasterol. The peak shapes and resolution were better with the phenyl
column, but despite the 80-minute run time, campesterol and stigmasterol
co-eluted. Quantitative results with the APCI MS detector were comparable to gas
chromatography with flame-ionization detection (GC-FID).55 Fibigr et al. achieved acceptable
separation of eight phytosterols, including campesterol, β-sitosterol, and
stigmasterol, on a narrow-bore RP-18 column in 8.5 minutes. However, the test
method was not applied to a saw palmetto extract.56 Establishing the presence of
ubiquitous phytosterols such as campesterol, β-sitosterol, and stigmasterol
does not provide a definitive means to detect adulteration. However, the
assessment of the phytosterol fingerprint as an approach for the detection of
saw palmetto adulteration could be useful as a complementary method in
evaluation of the extract authenticity. While none of the above methods have
measured phytosterols in adulterating vegetable oils, the qualitative and
quantitative sterol composition of these adulterating materials is well known. Some
of the “saw palmetto” samples containing animal fats have low (< 0.2%) amounts
of total sterols, but unusually high content of Δ5,24-stigmastadienol or Δ7-avenasterol. In addition,
these samples tend to have a low campesterol/stigmasterol ratio (0.78 – 1.67)
compared to authentic saw palmetto extracts (2.22-2.35).1 Compared to most GC-FID
methods, HPLC-MS has the advantage of a faster sample preparation since there
is no need to silylate the phytosterols after hydrolysis in potassium
hydroxide. However, the resolution of the sterols is generally better using
GC-FID.
Fatty
acid analysis: Al-Achi et al. analyzed fatty acids after a
conversion into fatty acid bromophenacyl esters using 2,4’-dibromoacetophenone
and dicyclohexano-18-crown-6 as catalyst, allowing the use of an ultraviolet
(UV) detector for quantification. Gradient conditions suggest a normal phase
separation, but neither the column packing nor the detection wavelength was indicated.
The method allowed for quantification of eight fatty acids in commercial saw
palmetto products.57 The omission of
important method information, lack of a chromatogram to assess the resolution
and peak shape, and absence of validation data means that the method cannot be
evaluated for its fitness to detect adulteration. Since validated GC methods
are available for fatty acid analysis (see below) with comparable time and
complexity requirements regarding sample preparation, these validated methods
are considered a better option for use in evaluating the authenticity of saw palmetto
extracts. In 2019, Marti et al. analyzed 35 samples of saw palmetto extract by
ultra high-performance liquid chromatography (UHPLC)-high-resolution MS. In
addition to the fatty acids, the authors determined the amounts of mono-, di-,
and triglycerides, and phosphorylated glycerides. Ethanol, hexane, and
CO2-extracts were readily distinguished using multivariate statistics (PCA, orthogonal partial least squares discriminant analysis
[OPLS-DA]).32 No adulterated samples were included in the analysis, but
based on the inclusion of a large number of saw palmetto constituents, and the
discriminatory power of the assay, this approach may be very useful in the
determination of saw palmetto authenticity.
11.2.4
Gas chromatography
Numerous methods described in the following literature
were evaluated in this review: Bedner et al.,55 Booker et al.,58 Mikaelian and Sojka,35 Ph. Eur. 9.1,17,29 Penugonda and
Lindshield,59 Priestap et al.,60 Sorenson and
Sullivan,61 Srigley and Haile,62 de Swaef and
Vlietinck,63 USP,18,33,64 and Wang et al.65
Comments: Gas
chromatography has been the method of choice to analyze fatty acids, fatty
alcohols, and phytosterols in saw palmetto extracts. The determination of the
qualitative and quantitative fatty acid content has been the major focus in the
analysis of saw palmetto extracts.
Fatty
acid analysis: Measuring fatty acids by GC is usually done
after converting the free and bound fatty acids into fatty acid methyl esters. An
exception is one of the methods by Priestap et al., where the fatty acids are
determined without derivatization using a nonpolar column (Table 3). While sample
preparation is quick and easy, the run time is long and the peaks are broader
and less well-resolved than those of the corresponding methyl esters.
Conversion into fatty acid methyl esters is done by methanolysis under acidic
or alkaline conditions,33,35,58,59,64 or by using specific methylation
reagents such as trimethylsulfonium hydroxide,17,29,63 diazomethane,60 or m-trifluoromethylphenyl trimethylammonium hydroxide.65
Methanolysis takes more time since it involves heating the samples for up to
two hours to complete the reaction. Some of the methylating reagents represent a
convenient alternative, but are considered more hazardous to health. Particular
caution should be used when using diazomethane due to its acute toxicity and
risk of explosion.
Separation of the fatty acid methyl esters
has been done on a number of stationary phases, with methylpolysiloxane-,
cyanopropyl-, or polyethylene-coated columns being the most commonly used. Run
times vary between 14 minutes35 and 66 minutes59 (see Table 3), not
including the time to re-establish initial temperature and column equilibration. Detection is achieved
by FID17,18,29,33,59,60,63,64 and/or MS.60,65 Chromatograms were presented in only two
publications: de Swaef and Vlietinck have a good separation of all the fatty
acid methyl and ethyl esters.63 In the case of Wang
et al., the peaks of linolenic and oleic acids overlap, and show an apparent
fronting.65
Table
3: Comparison among GC Methods to Determine Fatty Acids in Saw Palmetto Extracts.
aThe sample preparation time
is based on the reported duration of various sample preparation steps provided
in the experimental section of the corresponding paper and the estimated
duration of e.g., weighing, dilution, centrifugation, etc., listed in the ABC-AHP-NCNPR
Botanical Adulterants Prevention Program’s Skullcap Adulteration Laboratory Guidance
Document.66
Based on thorough validation
of GC methodology and easy sample preparation, the Ph. Eur. method is a good
choice for the analysis of saw palmetto fatty acids. Sample preparation time in
the USP method (Figure 5) is longer, but the shorter GC run time is
advantageous. In addition, USP has detailed a specific range for the ratio of nine
fatty acids relative to lauric acid, which can be used to detect adulteration
with vegetable oils, unless these are mixed in a way to mimic the saw palmetto
fatty acid composition. A simple additional sample preparation method for the
determination of free fatty acids in saw palmetto extract has been developed
and submitted in 2017 to USP as a Saw Palmetto Extract monograph revision.35 This method uses methanolic
sodium hydroxide to hydrolyze the mono-, di, and triglycerides in order to
selectively provide fatty acids methyl esters from fatty acids bound to
glycerin. Conversely, when methanolic sulfuric acid (or other strong acid) is
used for the reaction, methyl esters of both free and bound fatty acids are obtained.
By calculating the difference between
total and glycerin-bound concentrations for each individual fatty acid, the concentration
of free fatty acids can be determined.
Designer blends that are made with mixed
vegetable oils or fatty acids derived from animal fats may be present when
phytosterol or fatty alcohol concentrations are outside the specifications. In
other cases, the use of stable isotope measurements has proven helpful to
detect such fraud.1,12
Phytosterol
analysis: Due to the need for a hydrolysis step (some of the
phytosterols occur as fatty acid esters in the extract) with subsequent derivatization
with a silylating agent, the sample preparation for sterols is lengthy,
involving many manipulations. Hydrolysis is achieved by heating the sample in ~2M
potassium hydroxide solution. Sterols are either recovered by partitioning the
aqueous solution with toluene55,61 or diethyl ether,62 or by adsorbing the solution onto
diatomaceous earth, followed by elution with methylene chloride.29,33 Both Ph. Eur. and USP use the same
GC-FID method on a dimethylpolysiloxane column. The AOAC method (Sorenson and
Sullivan; Bedner et al.)55,61 and the method by Srigley and Haile62 use a phenylmethylpolysiloxane
stationary phase, although AOAC also permits a dimethylpolysiloxane column. Run
times are 33-66 minutes. The conditions established by Srigley and Haile, with
a run time of 66 minutes, allow quantification of up to 18 common phytosterols.
All methods provide a good separation of the saw palmetto phytosterols and have
been extensively validated. As mentioned in section 11.2.3 above, the analysis
of phytosterols as a stand-alone method is not sufficient to rule out
adulteration, but it is an excellent choice as a complementary method since
deviations from pharmacopeial specifications (not less than 0.2% total sterols,
not less than 0.1% β-sitosterol) are a good indication of ingredient
adulteration.
Fatty
alcohol analysis: USP is the only compendial standard to
measure fatty alcohols in saw palmetto extracts.33 Sample preparation
and analysis conditions are the same as for the sterols, which is convenient as
both classes of compounds can be measured in a single run. As with the sterol
analysis, the determination of fatty alcohols by itself is insufficient to
detect adulteration, but it is considered a valuable complementary means of
verifying the authenticity of saw palmetto extracts.
11.2.5
Nuclear magnetic resonance
Two methods described in the literature were evaluated in
this review: Booker et al.,58 and de Combarieu et
al.67 The NMR parameters
outlined by de Combarieu et al. were also used by Perini et al.1
Comments: Even
though the 1H NMR spectrum of saw palmetto extract is relatively
simple compared to extracts of other botanicals, a lot of useful information
can be obtained by visual evaluation of the spectrum. Adulteration with
vegetable oils can be readily distinguished by the presence of the signals of
the α/α' and β-protons of triglycerides (Figure 6), which are much smaller in
saw palmetto extracts than in vegetable oils.68 Using data from the
PCA loadings plot, Booker et al.58 and Perini et al.1 noticed that the
regions between 4.1-4.2 ppm, and between 5.3-5.5 ppm were important for
clustering of the samples (commercially available finished products). The
assessment of three principal components allowed for authentic saw palmetto to
be distinguished, even from animal fat-based ‘designer blends’ matching the saw
palmetto fatty acid profile.1 Based on all the data, 1H
NMR represents a valuable tool to detect saw palmetto adulteration, but is often
not part of the instruments found in a botanical ingredient or dietary
supplement manufacturing quality control laboratory.
11.2.6
Stable isotope ratio
Stable isotope analysis for the authentication of saw
palmetto extracts has been described in two separate publications by Perini et
al.1,12
Comment: Variations
in the stable isotopic ratios (SIRs) in plants and animals may occur for a
number of reasons. For example, the 2H/1H ratio in plants
is influenced by the geographical origin of the local water. The 13C/12C
ratio in plants depends on the type of photosynthesis that a plant utilizes.
While most plants exclusively use the Calvin cycle, some plants (e.g., corn [Zea mays, Poaceae] or sugar cane [Saccharum officinarum, Poaceae]) have
additional photosynthetic pathways, leading to a slightly higher 13C/12C
ratio in the latter. The 13C/12C isotopic ratio of animal
fats is known to be correlated with their diet, e.g., animals that feed exclusively
on corn will have a higher 13C/12C ratio than those that
ingest a wider variety of plants. The 18O/16O ratio
depends on the temperature, freshwater input, and other climatic factors. Results
are expressed as the ratio difference (δ2H, δ13C, δ18O)
of the material to be analyzed and a standard with a known isotopic ratio,
e.g., the Pee Dee Belemnite (based on a Cretaceous marine fossil
from the Pee Dee Formation
in South Carolina), which is one of the standards used for the 13C/12C
ratio, and the Vienna
Standard Mean Ocean Water (VSMOW), which defines the 2H/1H
and 18O/16O composition of fresh water.
Isotope ratios can be measured using gas chromatography
with an isotope mass spectrometer. In the approach by Perini et al., the
addition of a single-quadrupole mass spectrometer allowed identification of
individual compounds at the same time as the isotope ratios were measured. While
reported stable isotope ratios of some of the vegetable oils overlap with those
of saw palmetto, measuring the δ18O may provide valuable information
about the possible risk of adulteration since the δ18O of most
vegetable oils is lower than the range observed in saw palmetto. Fatty acids
derived from animal sources have a δ18O and a δ2H below those
reported for saw palmetto, and therefore can be readily detected as
adulterants, as evidenced in the publications by Perini et al.1,12
Measuring the stable isotope
ratios of bulk fatty oils is a helpful means to detect adulteration, especially
when a number of isotopes are measured and analyzed using appropriate
statistical tools. Further research needs to be done to verify the ability of
SIR analysis to detect other potential adulterants, and to determine the limit of
detection of this technique. Due to the availability and ease-of-use of more
established methods, the application of stable isotope analysis may be best
suited as an orthogonal assay to confirm adulteration, and to determine the
origin of the adulterant.
12.
Conclusion
Identification of saw
palmetto extract adulteration has been achieved using a number of analytical
techniques. Macroscopic and organoleptic assessment may provide the first
indication of adulteration by observing the color and strongly aromatic and
foul odor. Absence of the characteristic is a good indication that the oil is
adulterated. In practice, several assays are needed to confirm the authenticity
of saw palmetto extract. Gas chromatography for measuring fatty acid, fatty
alcohol, and phytosterol profiles, combined with a visual and organoleptic inspection
of the liquid and determination of the acid value, provides a robust affirmation
of saw palmetto extract authenticity. 1H NMR spectroscopy (with or
without chemometric data analysis) provides a suitable option for those
companies with access to an NMR instrument.
13. References
- Perini M, Paolini M, Camin F, et al.
Combined use of isotopic fingerprint and metabolomics analysis for the
authentication of saw palmetto (Serenoa
repens) extracts. Fitoterapia. 2018;127:15-19.
- Gafner S, Baggett S. Adulteration of saw palmetto (Serenoa repens), version 3. Botanical
Adulterants Prevention Bulletin. Austin, TX: ABC-AHP-NCNPR Botanical
Adulterants Prevention Program; 2018:1-7.
- McGuffin M, Kartesz JT, Leung AY, Tucker AO. Herbs of Commerce. 2nd ed. Silver Spring, MD: American Herbal
Products Association; 2000.
- Melzig MF, Hiller K, Loew D. Sabalis serrulatae fructus. In: Blaschek W,
ed. Wichtl — Teedrogen und Phytopharmaka.
Stuttgart, Germany: Wissenschaftliche Verlagsgesellschaft mbH; 2016:572-574.
- The Plant List. Version 1.1. http://www.theplantlist.org/tpl1.1/search?q=serenoa+repens.
Accessed June 12, 2017.
- Anderson MK, Oakes T. Plant guide for saw palmetto (Serenoa repens). Davis, CA: USDA-Natural Resources Conservation
Service, National Plants Data Team; 2012.
- Nelson G. The Shrubs and Woody
Vines of Florida: A Reference and Field Guide. Sarasota, FL: Pineapple
Press, Inc; 1996.
- Medicinal Plant Names Services (MPNS), Version 7.0 Royal Botanic Gardens,
Kew; 2017. http://mpns.kew.org/mpns-portal/?_ga=1.239114563.1577664092.1475222805.
Accessed June 6, 2017.
- The Plant List. Version 1.1 http://www.theplantlist.org.
Accessed May 19, 2017.
- 10. National Plant Germplasm System.
Germplasm Resources Information Network [Internet]. United States Department of
Agriculture, Agricultural Research Service. https://www.ars-grin.gov/npgs/index.html.
Accessed November 29, 2017.
- The Biology of Brassica napus L.
(canola/rapeseed). Canadian Food Inspection Agency; 2017. http://www.inspection.gc.ca/plants/plants-with-novel-traits/applicants/directive-94-08/biology-documents/brassica-napus-l-/eng/1330729090093/1330729278970.
Accessed February 28, 2019.
- Perini M, Paolini M, Pace R, Camin F. The use of stable isotope ratio
analysis to characterise saw palmetto (Serenoa
repens) extract. Food Chem. 2019;274:26-34.
- Hiermann A, Hübner WD, Schulz V. Serenoa. In: Hänsel R, Keller K, Rimpler
H, Schneider G, eds. Hager's Handbuch der
Pharmazeutischen Praxis. Drogen P-Z. Vol 2. Heidelberg, Germany: Springer
Verlag; 1994:680-687.
- Peng TS, Popin WF, Huffman M. Systematic investigation on quality
management of saw palmetto products. In: Ho CT, Zheng QY, eds. Quality Management of Nutraceuticals.
Vol 803. Washington, DC: American Chemical Society; 2002:117-133.
- Little DP, Jeanson ML. DNA barcode authentication of saw palmetto herbal
dietary supplements. Sci Rep. 2013;3:3518.
- Identifying commonly cultivated palms. Florida Department of Agriculture
and Consumer Service; 2011. http://idtools.org/id/palms/palmid/.
Accessed February 28, 2019.
- Sabalis serrulatae fructus. European
Pharmacopoeia (Ph. Eur. 9.1). Strasbourg, France: European Directorate for
the Quality of Medicines and Health Care; 2014:1512-1513.
- Saw palmetto. USP 41-NF 36.
Rockville, MD: United States Pharmacopeial Convention; 2018:4856-4858.
- Fructus Serenoae repentis. WHO Monographs
on Selected Plants. Vol 2. Geneva, Switzerland: World Health Organization;
2002:285-299.
- 20. Henderson A, Galeano G, Bernal
R. Field Guide to the Palms of the
Americas. Princeton, NJ: Princeton University Press; 1995.
- Zona S. The genera of Palmae (Arecaceae) in the southeastern United
States. Harvard Papers in Botany. 1997;2:71-107.
- Upton R, Graff A, Jolliffe G, Länger R, Williamson E. American Herbal Pharmacopoeia: Botanical
Pharmacognosy—Microscopic Characterization of Botanical Medicines. Boca
Raton, FL: CRC Press; 2011.
- Barrett CF, Baker WJ, Comer JR, et al. Plastid genomes reveal support for
deep phylogenetic relationships and extensive rate variation among palms and
other commelinid monocots. New Phytol. 2016;209(2):855-870.
- Barrett CF, Bacon CD, Antonelli A, Cano Á, Hofmann T. An introduction to
plant phylogenomics with a focus on palms. Bot
J Linnean Soc. 2016;182(2):234-255.
- Couvreur TL, Forest F, Baker WJ. Origin and global diversification
patterns of tropical rain forests: inferences from a complete genus-level
phylogeny of palms. BMC Biology. 2011;9(1):44.
- Baker WJ, Savolainen V, Asmussen-Lange CB, et al. Complete generic-level
phylogenetic analyses of palms (Arecaceae) with comparisons of supertree and
supermatrix approaches. Syst Biol. 2009;58(2):240-256.
- Joint WHO/FAO Codex Alimentarius Commission. Codex Alimentarius: Standard
for named vegetable oils. Vol CODEX STAN 210-1999. Rome, Italy: World Health
Organization and Food and Agriculture Organization of the United Nations;
2015:1-13.
- Harbaugh Reynaud DT. The DNA toolkit: a practical user's guide to genetic
methods of botanical authentication. In: Reynertson K, Mahmood K, eds. Botanicals. Boca Raton, FL: CRC Press;
2015:43-68.
- Sabalis serrulatae extractum. European
Pharmacopoeia (Ph. Eur. 9.1). Strasbourg, France: European Directorate for
the Quality of Medicines and Health Care; 2014:1509-1511.
- Mikaelian G, Hill WS, Nguyen U, Holzer SJ. Preliminary quality
examination of saw palmetto extract. Nutra
Bus Technol. 2006;2:64-65.
- Olennikov DN, Zilfikarov IN, Khodakova SE. Phenolic compounds from Serenoa repens fruit. Chem Nat Compd. 2013;49(3):526-529.
- Marti G, Joulia P, Amiel A, et al. Comparison of the phytochemical
composition of Serenoa repens
extracts by a multiplexed metabolomic approach. Molecules. 2019;24(12):2208.
- Saw palmetto extract. USP 41-NF 36.
Rockville, MD: United States Pharmacopeial Convention; 2018:4860-4861.
- Mikaelian G, Sojka M, Minatelli J. The ultimate way to win the fight
against saw palmetto extract adulteration. Nutra
Bus Technol. 2009;1:46-50.
- Mikaelian G, Sojka M. Authenticating saw palmetto extract : a new
approach. Nutra Bus Technol. 2009;5:24-27.
- Schantz MM, Bedner M, Long SE, et al. Development of saw palmetto (Serenoa repens) fruit and extract
standard reference materials. Anal
Bioanal Chem. 2008;392(3):427-438.
- Giammarioli S, Boniglia C, Di Stasio L, Gargiulo R, Mosca M, Carratù B.
Phytosterols in supplements containing Serenoa
repens: an example of variability of active principles in commercial plant
based products. Nat Prod Res. 2019;33(15):2257-2261.
- Ham B, Jolly S, Triche G, Williams PR, Wallace F. A study of the physical
and chemical properties of saw palmetto berry extract. Chemistry Preprint Archive. 2002(2):106-121.
- Suzuki M, Ito Y, Fujino T, et al. Pharmacological effects of saw palmetto
extract in the lower urinary tract. Acta
Pharmacol Sin. 2009;30(3):227-281.
- O'Brien RD. Fats and Oils:
Formulating and Processing for Applications. 3rd ed. Boca Raton, FL: CRC
Press; 2009.
- Gunstone FD, Harwood JL, Dijkstra AJ. The
Handbook of Lipids. Boca Raton, FL: CRC Press; 2007.
- Przbylski R, Mag T, Eskin NAM, McDonald BE. Canola oil. In: Shahidi F,
ed. Bailey's Industrial Oil and Fat
Products. Vol 2. 6 ed. Hoboken, NJ: John Wiley & Son, Inc.; 2005.
- Orsavova J, Misurcova L, Ambrozova JV, Vicha R, Mlcek J. Fatty acids
composition of vegetable oils and Its contribution to dietary energy intake and
dependence of cardiovascular mortality on dietary intake of fatty acids. Int J Mol Sci 2015;16(6):12871-12890.
- Siew WL, Ng W-L. Diglyceride content and composition as indicators of
palm oil quality. J Sci Food Agric. 1995;69(1):73-79.
- Warner K, Vick BA, Kleingartner L, Isaac I, Doroff K. Composition of
sunflower NuSun (mid-oleic sunflower), and high-oleic sunflower oils. Paper
presented at: Sunflower Research Workshop.2003; Fargo, ND.
- International Olive Council. Trade standard applying to olive oils and
olive pomace oils. Vol COI/T.15/NC No 3/Rev. 12. Madrid, Spain: International
Olive Council; 2018:17.
- Yorulmaz A, Erinc H, Tekin A. Changes in olive and olive oil
characteristics during maturation. J Am
Oil Chem Soc. 2013;90(5):647-658.
- Boskou D, Blekas G, Tsimidou M. Chemistry, properties, health effects.
In: Boskou D, ed. Olive Oil: Chemistry
and Technology. 2 ed. Champaign, IL: AOCS Press; 2006:41-72.
- Vulcano I, Halabalaki M, Skaltsounis L, Ganzera M. Quantitative analysis
of pungent and anti-inflammatory phenolic compounds in olive oil by capillary
electrophoresis. Food Chem. 2015;169:381-386.
- Cicerale S, Conlan XA, Sinclair AJ, Keast RSJ. Chemistry and health of
olive oil phenolics. Crit Rev Food Sci
Nutr. 2008;49(3):218-236.
- Serenoa repens, fruit. HPTLC
Association; 2019. Accessed August 8, 2019.
- Halkina T, Sherma J. Determination of sterols and fatty acids in prostate
health dietary supplements by silica gel high performance thin layer
chromatography with visible mode densitometry. J Liq Chromatogr Relat Technol. 2007;30(15):2329-2335.
- Hanson BA, Ye T, Raftery DM. Assessing Serenoa repens (Arecaceae) quality at the retail level using
spectroscopic and chemometric methods. The 49th Annual Meeting of the Society
for Economic Botany; 2008; Durham, NC.
- Villar A, Mulà A. Full traceability, high quality production and exhaustive
analytical control – industry’s key tools to avoid and prevent adulteration and
fraud of botanical ingredients. Adulteration and Fraud of Botanical and Natural
Health Ingredients: Issues, Challenges and Prevention Tools for the Industry;
2018; Frankfurt, Germany.
- Bedner M, Schantz MM, Sander LC, Sharpless KE. Development of liquid
chromatographic methods for the determination of phytosterols in Standard
Reference Materials containing saw palmetto. J Chromatogr A. 2008;1192(1):74-80.
- Fibigr J, Šatínský D, Solich P. A UHPLC method for the rapid separation
and quantification of phytosterols using tandem UV/Charged aerosol detection –
A comparison of both detection techniques. J
Pharm Biomed Anal. 2017;140:274-280.
- Al-Achi A, Locklear AF, Fetterman L. Commercially available saw palmetto
products: Quality control testing. Int J
Drug Discovery Herbal Res. 2012;2(1):267-271.
- Booker A, Suter A, Krnjic A, et al. A phytochemical comparison of saw
palmetto products using gas chromatography and (1)H nuclear magnetic resonance
spectroscopy metabolomic profiling. J
Pharm Pharmacol. 2014;66(6):811-822.
- Penugonda K, Lindshield BL. Fatty acid and phytosterol content of
commercial saw palmetto supplements. Nutrients.
2013;5(9):3617-3633.
- Priestap H, Houle P, Bennett B. Fatty acid composition of fruits of two
forms of Serenoa repens. Chem Nat Compd. 2011;47:511-514.
- Sorenson WR, Sullivan D. Determination of campesterol, stigmasterol, and
beta-sitosterol in saw palmetto raw materials and dietary supplements by gas
chromatography: single-laboratory validation. J AOAC Int. 2006;89(1):22-34.
- Srigley CT, Haile EA. Quantification of plant sterols/stanols in foods
and dietary supplements containing added phytosterols. J Food Comp Anal. 2015;40:163-176.
- De Swaef SI, Vlietinck AJ. Simultaneous quantitation of lauric acid and
ethyl laurate in Sabal serrulata by
capillary gas chromatography and derivatisation with trimethyl
sulphoniumhydroxide. J Chromatogr A. 1996;719:479-482.
- Powdered saw palmetto. USP 41-NF 36.
Rockville, MD: United States Pharmacopeial Convention; 2018:4858-4860.
- Wang M, Avula B, Wang Y-H, Zhao J, Parcher JF, Khan IA. Fatty acid
analysis of saw palmetto (Serenoa repens)
and pygeum (Prunus africana) in dietary
supplements by gas chromatography/mass spectrometry in the selected ion
monitoring mode. J AOAC Int. 2013;96(3):560-566.
- Gafner S. Skullcap adulteration laboratory guidance document. Austin, TX:
ABC-AHP-NCNPR Botanical Adulterants Prevention Program; 2015:1-12.
- De Combarieu E, Martinelli EM, Pace R, Sardone N. Metabolomics study of
saw palmetto extracts based on 1H NMR spectroscopy. Fitoterapia. 2015;102:56-60.
- Gafner S, Blumenthal M, Foster S, Cardellina II JH, Khan IA, Upton R.
Botanical ingredient adulteration – how some suppliers attempt to fool commonly
used analytical techniques. Acta Hort. 2019:in
press.