|
Marketing botanical ingredients as foods
and dietary ingredients in the United States is commonplace. Getting them
approved as prescription drugs is a somewhat new frontier, and so far only two
botanicals have achieved this goal. On October 31, 2006, the US Food and Drug
Administration (FDA) approved the first botanical drug, Veregen® (sinecatechins; ointment, 15%; Medigene, Planegg/Martinsried,
Germany), a proprietary extract of green tea (Camellia sinensis Kuntze) for treating genital and perianal warts.1
FDA approved the second botanical New Drug Application (NDA) on December
31, 2012, for FulyzaqTM (crofelemer; 125
mg tablet; Salix Pharmaceuticals, Raleigh, North Carolina), the first oral
prescription botanical drug for the novel indication of HIV-associated
diarrhea. Fulyzaq is a proprietary extract of the blood-red latex of the South
American croton tree (Croton lechlerii Müll. Arg).2, 3
Defining “Botanical
Drug”
FDA defines a “botanical” as a finished product
containing ingredients and/or constituents of vegetable matter. This classification
includes whole plants or plant parts — including plant materials such as
juices, gums, fatty oils, scent oils, etc. — and also includes algae or
macroscopic fungi and similar products. Excluded are fermentation products,
isolated and purified ingredients, or homeopathic ingredients, all of which already
have well-described drug regulatory pathways in the United States.4
Because both Fulyzaq and Veregen are intended to diagnose, treat, prevent,
mitigate, or cure an abnormal condition, they are considered “drugs.” In
particular, they are “new” drugs, i.e.,
drugs marketed in the United States after 1938 that prior to approval were “not generally recognized as safe and effective under the
conditions prescribed, recommended, or suggested in the labeling.”5 As new drugs, the sponsors were required to
submit NDAs for FDA pre-market approval. Each product underwent
extensive product and clinical development to meet the drug requirements and to
document safety and efficacy for its intended use(s). Unlike foods, dietary
supplements, or cosmetic products, which are restricted from making disease claims,
these botanical drugs can make disease claims that are supported by their
approved NDAs. Both products are
available only by prescription.
Initial Steps: Investigational
New Drug (IND) Application
Botanical drug development begins long
before FDA’s review process. A botanical must undergo identification and
taxonomic classification. Raw material sourcing as well as collection,
manufacturing, and formulation practices must be described adequately. Details
of any prior and current human use also are important to obtain, as such
information can significantly impact the regulatory requirements.4
After these critical steps, filing an Investigational
New Drug application (IND) with FDA is the first part of the formal drug regulatory
process that culminates with FDA’s decision on the NDA. An IND exempts the “investigational
new drug” from federal requirements that it must be safe and effective, allowing
for research and development activities to take place within US borders.6
Following an initial submission, FDA has 30
calendar days to review the IND. For early (Phase 1) clinical development, FDA focuses
on the drug’s safety, which is based on its chemistry, toxicity profile, and
use history. Novel chemicals submitted under IND require extensive nonclinical toxicology
work, even for Phase 1. A botanical drug with a well-documented history of
human use can often circumvent much of the toxicology requirements, at least
initially, although some safety testing is generally required.4
Ultimate Step: New
Drug Application (NDA)
Because
new drugs must undergo FDA pre-market approval, NDA submission is the ultimate step
in the development process.7 The NDA is a collation of data and
analyses collected under the IND, a summary of which will become the drug package insert
to support drug labeling and promotion. Requesting a “pre-NDA” meeting with FDA
helps drug sponsors ascertain whether FDA agrees with their
marketing proposals. FDA expects to meet with the NDA sponsor to discuss the content
and format of the application prior to its submission, as well as any unresolved
issues raised during the IND, and any further requirements for potential
approval. This may include completion of nonclinical testing, pivotal trials
analyses, and submission of key clinical study databases for FDA’s own review
and analysis. The sponsor also will need to submit the status of any unexpired patents
for the drug.8 FDA even has final say over the drug’s brand and
scientific (“generic”) names.9
The most important step of
the process and, for botanicals, the most difficult step to satisfy is FDA’s review
and acceptance of the chemistry, manufacturing, and controls (CMC). At the initial
development stages (Phase 1 and early Phase 2), CMC requirements are relaxed.
For example, FDA often allows the current nondrug formulation to be used in early-phase
studies. To support the NDA, however, later-stage (i.e., Phase 2b and pivotal*) clinical studies must be conducted using
finished drug product that conforms to pharmaceutical requirements (Good
Manufacturing Practices). FDA also
will want to review and negotiate the sponsor’s plans for commercialization,
including manufacturing scale-up, packaging, and lot-release protocols to be
utilized in the commercial production of the drug following approval. Finally,
the sponsor must be prepared for FDA to conduct a pre-approval inspection of
the manufacturing facilities.
NDA Approval
Requirements FDA can approve the NDA when the drug meets
the legal requirements discussed herein. Federal law requires that a new drug
be safe and effective for its intended use, as demonstrated by substantial evidence, defined as “evidence consisting of
adequate and well-controlled investigations, including clinical investigations,
by experts qualified by scientific training and experience to evaluate the
effectiveness of the drug involved.” Additionally, data from the substantial
evidence demonstration must show that the “drug will have the effect it
purports or is represented to have under the conditions of use prescribed,
recommended, or suggested in the labeling….”5 Control over the
drug’s lot-to-lot variation also must be adequately addressed.7
Botanical drug approval is
a very different process from acceptance of the same ingredient as a nondrug.
For example, foods and dietary supplements are allowed to be marketed if they
are food ingredients or ingredients that are “generally
recognized as safe” (GRAS),
or contain ingredients with “a history of use or other evidence of safety” that “will reasonably be expected to be safe…”5
In contrast, a drug must demonstrate that its benefits outweigh its risks
to the population for which its use is intended.
NDA Review Timeline and the “Prescription Drug User Fee Act”
(PDFUA) FDA’s timelines for NDA reviews are
guided by the current Prescription Drug User Fee Act (PDUFA). Originally enacted
in 1992 by the US Congress to reduce lengthy NDA review times, PDUFA authorizes
FDA to collect fees from sponsors whose applications require Agency review. “PDUFA
fees” support the Agency’s review processes by allowing the hiring of experts
and other activities. No fees are required if the NDA sponsor and/or product
fits one or more of the following scenarios: first-time filer, product for a
rare disease or condition under an Orphan Product Designation, product deemed
necessary to protect the US public health, product for which user fees will
pose a significant barrier to innovation due to limited resources or other
circumstances, or a sponsor that is a small business (<500 employees) that
does not yet have an approved drug on the US market.10 Upon
receipt of an NDA, FDA has 60 days to review and accept it for filing.
Incomplete or poorly organized applications can result in a “failure to file”
notice. FDA’s review “clock” does not start until the Agency allows the NDA to
be filed (referred to as the “acceptance to file” notification; see Figure 1). Once the NDA is filed, the
Agency sets a “goal” (PDUFA) date — the date by which FDA should act on the application
(see Table 1).
Figure 1 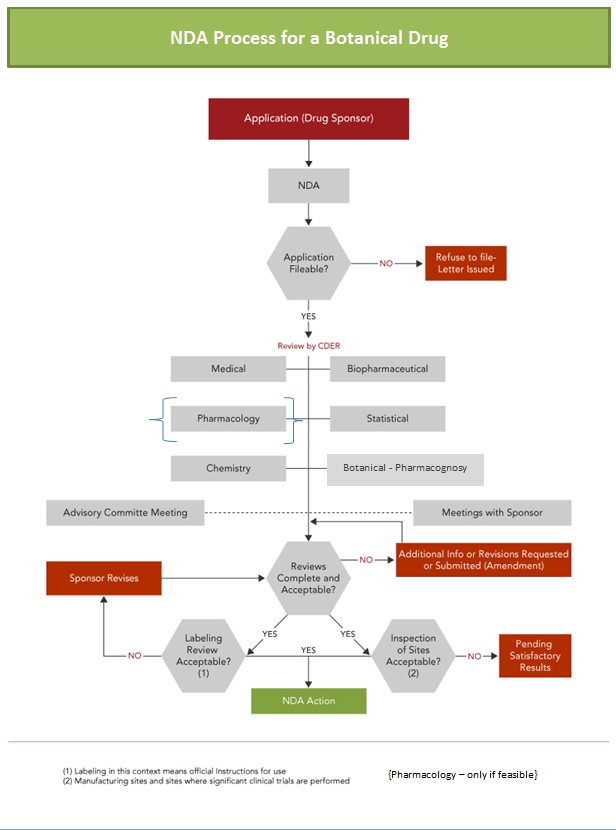
Adapted from: NDA Review Process (www.fda.gov/cder/handbook/)
FDA can modify the review timeline.
It can hold the clock while waiting for a response to its request for
information. Or, the timeline can be condensed when FDA assigns “Priority
Review” to NDAs for drugs intended to treat a serious and life-threatening condition
lacking satisfactory treatments. FDA also can utilize an “Accelerated Approval”
process to decide on a drug prior to receiving all safety or efficacy data
needed for approval. Although a drug can be marketed following Accelerated
Approval, its sponsor will be required to collect and to submit additional
efficacy and safety data at a later time for FDA to determine if the drug
should remain on the market. If these data do not continue to support the
drug’s safety or efficacy, FDA can revoke the approval.11
Table
1: FDA Review Timelines (under PDUFA V) for review of New Drug Applications
(NDAs) and Biologic Licensing Applications (BLAs)
On
July 9, 2012, President Obama signed into law the fifth reauthorization of the
Prescription Drug User Fee Act. Known as
“PDUFA V,” the new law took effect on October 1, 2012. It includes the
following timeframes used by FDA to project a calendar day (called the “PDUFA
date”) — a goal, but not a deadline —
by which it plans to return a decision, following the Agency’s review of the various
types of marketing applications.
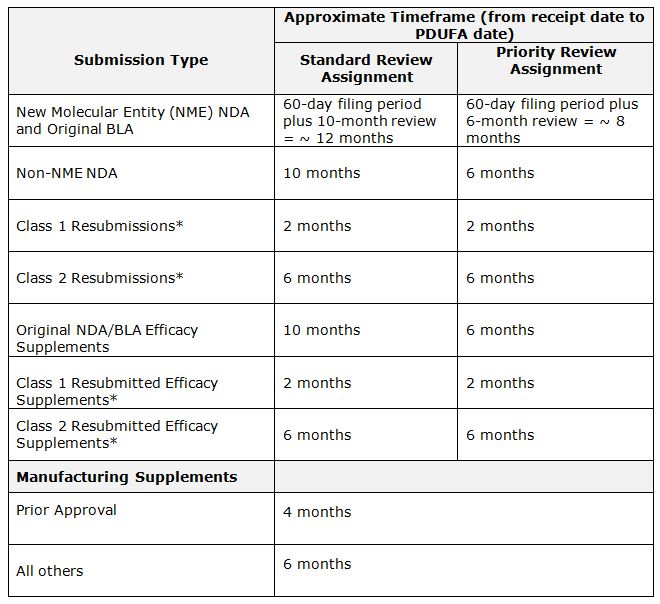
*
Resubmissions of an NDA or BLA are classified by the information provided by
the Sponsor to an FDA Action letter. These are further defined in FDA Guidance
for Industry — Classifying Resubmissions in Response to Action Letters — April
1998. [Editor’s Note: A delay in the PDUFA “goal” timeline
can arise from any of the scientific or regulatory areas that are required for
review (chemistry, nonclinical, clinical data, name selection, facility audits,
negotiations on the package insert, etc.), as discussed above. (http://www.fda.gov/Drugs/ResourcesForYou/Consumers/ucm289601.htm)]
Botanical Drug
Approvals
More than 500 pre-IND meetings and IND
applications have been submitted to FDA for botanical drugs; two botanical drug
NDAs have been submitted to FDA and both were approved (see Figure 2). While it appears that many
sponsors have accomplished the IND step, only two have reached the final NDA
step. Why are there only two FDA-approved NDAs? Based on these authors’ experience,
the following represent three of the most common reasons that could explain why
more botanical drug NDAs have not been submitted to FDA for review: · Failure to show efficacy Failure to show clinically relevant and statistically significant efficacy is the single most common reason why most drugs — not just botanical drugs — fail to reach the NDA step. Although many sponsors “believe” that their product “works,” the stringent criteria for US drug approval consist of documented safety and efficacy from one or more multicenter “adequate and well-controlled” clinical studies. For pivotal studies (those efficacy studies that will be used to support the NDA), it is very important that target populations be well-circumscribed by the protocol eligibility criteria, with appropriate and FDA-agreed upon outcome measures, proper controls (e.g., placebo or active treatment), and be well-monitored and accurately analyzed. · Unrealistic Expectations Inexperienced drug sponsors often have unrealistic expectations when it comes to planning and executing a drug development program. This may be due, in part, to FDA’s relatively relaxed requirements during initial stages of IND development, which may give sponsors a false sense of security that the requirements for botanicals are less rigorous than those for non-botanical drugs. It also may be due to the fact that regulatory requirements for botanicals are not internationally harmonized, as they are for other drug categories, which creates confusion, because US requirements differ from those of other countries. Also, many botanical drug sponsors have never developed a drug for the US market, or come from different industries or regulatory environments. Some sponsors are unwilling to accept — or simply deny — that the United States requires submission of “raw” data (chemistry, nonclinical safety testing, clinical study databases, etc.) to support drug filings, rather than data summaries or “expert” opinion, as is commonplace in other countries. · Insufficient Funding Lack of or insufficient funding to complete the development process is not an uncommon problem for many botanical drugs under IND. This may be due to the economic climate, lack of acceptance by the investment community, lack of patent status (although the product may enjoy other forms of intellectual property that may be superior to patents), or insufficient planning. Again, many botanical drug sponsors, particularly those whose products are in other market channels (e.g., dietary supplements), or foreign markets, underestimate the level of documentation and data that FDA requires to assess that a drug does what it claims to do in its labeling.
Figure 2 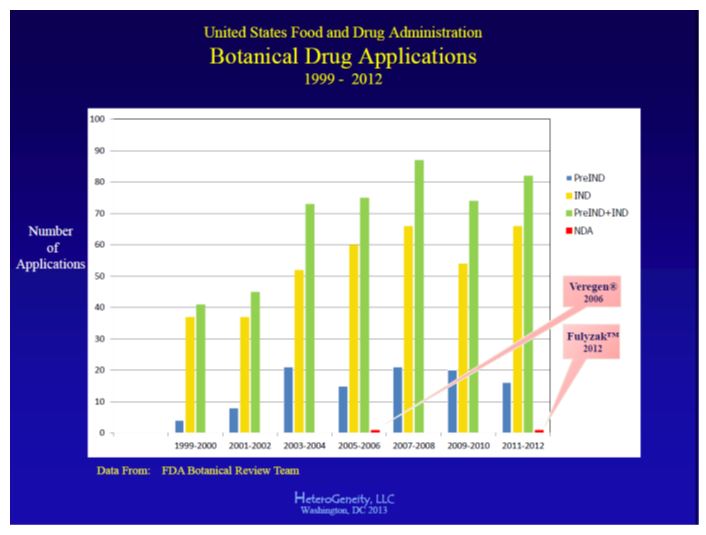
Conclusion
For many botanical drugs, the path to NDA approval has the potential to be shorter and less costly than for “standard” new chemical entities. However, until the botanical community comes to grips with the realities of the legal requirements for drug approval in the United States, there will continue to be few sponsors that are able to traverse this final frontier. *The term pivotal study refers to an adequate and well-controlled clinical trial designed to evaluate the specific dose, route, schedule, formulation, and specific clinical indication that will become the subject of the NDA. In particular, the drug product used for a pivotal study should meet FDA requirements for commercial manufacturing (Good Manufacturing Practices or GMPs).
—Freddie Ann Hoffman, MD1 and Steven R. Kishter, MD, DDS2
1 HeteroGeneity, LLC – Washington, DC (www.heterogeneity-LLC.com)
2 Avenue 16 Group – Washington, DC (www.ave16.com)
References
1. FDA approves special green tea extract as a new topical
drug for genital warts [press release]. Austin, TX: American Botanical Council;
November 9, 2006. Available here.
Accessed April 30, 2013.
2. FDA approves first anti-diarrheal
drug for HIV/AIDS patients [press release]. Silver Spring, MD: US Food and Drug Administration; December 31, 2012.
Available here.
Accessed April 30, 2013.
3. FDA approves crofelemer as first-ever oral botanical
drug [press release]. Austin, TX: American Botanical Council; January 2, 2013.
Available here.
Accessed April 30, 2013.
4. Guidance for Industry on Botanical Drugs
Products. US Department of Health and Human Services. US Food and Drug
Administration, Center for Drug Evaluation and Research. June 2004. Available here.
Accessed April 30, 2013.
5. Federal Food, Drug and Cosmetic Act and
its Amendments, 21 USC Chapter 9 §§ 301–399f.
6. Investigational New Drug application. US Food and
Drug Administration website. Available here. Accessed
April 30, 2013.
7. New
Drug Application. US Food and Drug Administration website. Available here. Accessed
April 30, 2013.
8. Applications For FDA Approval To Market A New Drug. Title
21 CFR §314.50.
9. Guidance for Industry: Contents of a Complete
Submission for the Evaluation of Proprietary Names. US Department of Health and
Human Services. Food and Drug Administration, Center for Drug Evaluation and
Research (CDER), Center for Biologics Evaluation and Research (CBER). February
2010. Available here.
Accessed April 30, 2013.
10. Prescription Drug User Fee Act (PDUFA). US Food
and Drug Administration website. Available here. Accessed April 30,
2013.
11. FDA
Commissioner removes breast cancer indication from Avastin label [news release].
Silver Spring, MD: US Food and Drug Administration; November 18, 2011.
Available here.
Accessed April 30, 2013.
|