The National Animal Supplement Council (NASC)
is a California-based nonprofit industry advocacy and educational group that is
dedicated to protecting and enhancing the health of companion animals (dogs,
cats, and horses) in the United States.
Founded in 2002, the NASC is made up of
more than 140 businesses that are committed to providing  high-quality health
supplements and nutritional supplements for these animals. Health supplements,
such as those that contain glucosamine, chondroitin sulfate,
methylsulfonylmethane (MSM), parsley (Petroselinum
crispum, Apiaceae), and peppermint (Mentha
× piperita, Lamiaceae), are
intended to support maintenance of normal biological structures and functions. Nutritional
supplements, such as those that contain vitamins and minerals, are intended to
provide nutritional value as a component of a complete and balanced diet.1,2 high-quality health
supplements and nutritional supplements for these animals. Health supplements,
such as those that contain glucosamine, chondroitin sulfate,
methylsulfonylmethane (MSM), parsley (Petroselinum
crispum, Apiaceae), and peppermint (Mentha
× piperita, Lamiaceae), are
intended to support maintenance of normal biological structures and functions. Nutritional
supplements, such as those that contain vitamins and minerals, are intended to
provide nutritional value as a component of a complete and balanced diet.1,2
NASC members are located around the world,
including in the United States, Canada, Europe, Australia, and China. An NASC
Primary Supplier Member must be a manufacturer, formulator, bottler, labeler,
or re-packer that markets its brand as the supplier of record. An NASC
Associate Member may be involved in selling or distributing animal
health/nutritional supplements as a distributor, dealer, retailer, veterinarian,
or internet/catalog company.
All NASC members are vetted in a direct
interview that presents and clarifies the requirements of the organization and
the standards to uphold. In addition, most NASC members complete an independent
NASC quality audit that, when passed, enables them to display the NASC
Quality Seal on their packaging and marketing materials. NASC membership demonstrates
a commitment to upholding specific quality standards, which the NASC hopes, in
turn, will increase consumer confidence in the animal  supplement industry.2 supplement industry.2
Preferred Supplier
Program Overview
The NASC Preferred Supplier Program is a
self-regulatory program that was initiated to help extend responsibility,
accountability, and uniformity upstream to the beginning of the animal
supplement supply chain. It also helps NASC members identify reputable suppliers
of reliable raw materials. “With the increased scrutiny on suppliers and supply
chain management, our intention has been, always, to keep our members ahead of
the regulatory curve,” said Bill Bookout, president of the NASC and chair of
its board of directors (oral communication, August 31, 2016).
Through data sharing, all involved parties
save time and money through the NASC’s Preferred Supplier Program. Suppliers
avoid having to submit to audits for every customer, and NASC members do not
have to go through the process of qualifying each supplier. Thus, all parties
contribute to the program and all parties benefit from the program, according
to Bookout.
The program qualifies suppliers in four
categories: Raw Material Suppliers, Contract Manufacturers (many NASC member
companies that market their own brand of products rely on one or more
third-party manufacturers to produce their products), Laboratories and
Research, and Service Providers (e.g., insurance providers, packagers or providers
of packaging components, legal service providers, web service providers, etc.).
So, in the context of the NASC’s program, the term “Preferred Supplier”
encompasses a wide variety of product and service providers along the supply
chain and is not limited only to suppliers of raw materials.3
There is an annual fee for each company in
the Preferred Supplier Program, and this covers the posting of all Preferred
Supplier information in the Members Section of the NASC’s website, the posting
of a company sales profile for all Preferred Suppliers, the opportunity to
conduct webinars and/or educational programs for NASC members, the availability
of contacts at NASC member companies, attendance at the Preferred Suppliers
Only Meeting during the NASC Annual Conference, and the opportunity to provide
input about the program.
Raw Material Suppliers and Contract
Manufacturers must complete and submit a Preferred Supplier Data Sheet, which
includes information about the manufacturing facility, testing information,
current Good  Manufacturing Practice (cGMP) compliance details, and more. In
addition, Raw Material Suppliers must complete and submit a Non-Botanical
Ingredient Data Sheet or a Botanical Ingredient Data Sheet for each unique ingredient
that the company supplies. Manufacturing Practice (cGMP) compliance details, and more. In
addition, Raw Material Suppliers must complete and submit a Non-Botanical
Ingredient Data Sheet or a Botanical Ingredient Data Sheet for each unique ingredient
that the company supplies.
These data sheets are modeled after the
data sheets of the Standardized Information on Dietary Ingredients (SIDI)
protocol, but have been adapted for the animal supplement industry. The SIDI
initiative is a voluntary, industry-wide protocol intended to standardize and
streamline communication of information about dietary ingredients from raw
material suppliers to finished product manufacturers. It is a cooperative
effort among three of the leading trade associations for the dietary supplement
industry: the Consumer Healthcare Products Association (CHPA), the Council for
Responsible Nutrition (CRN), and the United Natural Products Alliance (UNPA).4
For each ingredient that is qualified by
the NASC’s program, five batch/lot numbers (from one supplier) of that
ingredient must be tested to verify the information on the respective
certificate of analysis (COA) that accompanies each batch/lot. A COA is the
supplier’s test results for the batch/lot of raw material being supplied. The
information provided on the COA depends on the raw material, but it usually
includes information about quality, strength, purity, and composition. It may
also include notation of the plant part(s) used (for botanical ingredients), the
geographical source of the raw material, concentrations of marker compounds, and
levels of potential contaminants (if detected), including microbial (e.g., Salmonella and Escherichia coli) count and heavy metal (e.g., lead, mercury,
cadmium, and arsenic) count. In the case of boswellia (Boswellia spp., Burseraceae), for example, the COA could include
boswellic acid content. For garlic (Allium
sativum, Amaryllidaceae), it could include allicin yield. Methods of
analysis are also noted.
Samples from the first two batch/lot
numbers may be tested by the Raw Material Supplier from their own in-house laboratory
or submitted by the Raw Material Supplier for testing by an NASC-approved
laboratory. However, samples from the other three batch/lot numbers must be
submitted either by an NASC member that sources the ingredient from that
supplier, or by another customer of that supplier that uses the ingredient in
its product formulations, for testing by an independent, third-party, NASC-approved
analytical laboratory in the United States. The three independent tests, which
are conducted at the expense of the supplier, break the chain of custody and
eliminate sample bias. These tests may be performed by the laboratories that
are listed as Preferred Suppliers on the NASC’s website, in addition to other
reputable laboratories in the industry.
All raw materials must be tested using
methodology that follows current established recommendations of the United
States Pharmacopeia (USP), AOAC International, other recognized testing
authorities, published monographs (including monographs of the American Herbal
Pharmacopoeia [AHP]), or other published testing methods. Each raw material
qualified by the NASC’s program is subject to re-verification with one random
test performed annually.
Raw Material Suppliers must meet these
requirements for each individual ingredient in order for those ingredients to
be qualified by the NASC’s program, but there is no additional fee for qualifying
additional ingredients 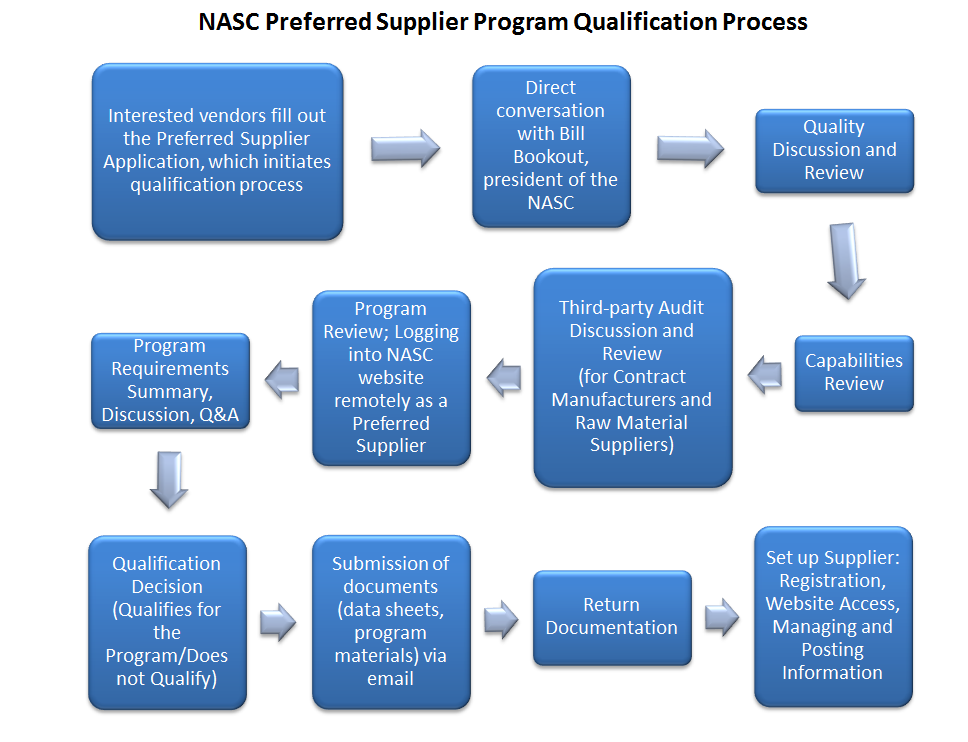
For Contract Manufacturers and Raw Material
Suppliers, the NASC will recognize facility audits that have been conducted by
NSF International, the Safe Quality Food (SQF) Institute, Underwriters
Laboratories (UL), the Natural Products Association (NPA), and other auditing bodies
that are accredited to confirm that manufacturing facilities are operating in
accordance with dietary supplement cGMPs found in 21 CFR (Code of Federal
Regulations) Part 111, which is called “the cGMP rule.” The cGMP rule requires
“persons who manufacture,
package, label, or hold a dietary supplement to establish and follow current
good manufacturing practice(s) to ensure the quality of the dietary supplement
and to ensure that the dietary supplement is packaged and labeled as specified
in the master manufacturing record.”5
The NASC requires Contract Manufacturers and Raw Material Suppliers to have
a current audit certificate. Typically, these certificates expire every one or
two years. If a Contract Manufacturer has not been audited, the NASC can conduct
a facility audit, but this takes more time.
Laboratories seeking qualification by the NASC’s program must complete and
submit an Analytical Laboratory Data Sheet. These laboratories must comply with
Good Laboratory Practices (GLPs) and good analytical methodology (as
recommended by USP, AOAC International, etc.). In addition, Service Providers
must complete and submit a Service Provider Data Sheet.
The data sheets submitted by all of the Preferred Suppliers, along with the
required testing information and any additional documentation (e.g., kosher statement,
hormone statement, sterilization methods [irradiation, ethylene oxide]
statement, genetically modified organism statement, and organic certification statement, etc.) are available for NASC members to download on the NASC website. In
addition, Preferred Suppliers may choose to post liability insurance
certificates.
Preferred Suppliers, however, are not required to post confidential
information to the NASC website. This includes proprietary processes or
anything covered under trade secrets. Preferred Suppliers may choose to make
certain information available to the NASC under a non-disclosure agreement
(NDA). Additionally,  distributors are not required to disclose their suppliers,
since manufacturers could then cut them out and go directly to the supplier,
but distributors must be able to verify how their suppliers were qualified. distributors are not required to disclose their suppliers,
since manufacturers could then cut them out and go directly to the supplier,
but distributors must be able to verify how their suppliers were qualified.
Ingredients that have been qualified by the
program are searchable alphabetically by common name on the NASC website. Each common
name listing includes all of the Raw Material Suppliers that have been
qualified to supply that particular ingredient, in addition to the required
testing information and additional documentation.
Conclusion
Currently, more than 60 Preferred
Suppliers, some of which are also NASC members and most of which are located in
the United States, have been qualified by the NASC’s program. These Preferred
Suppliers are able to display the blue NASC Preferred Supplier Seal. “Our goal
with the program is not to get every single company that possibly participates
in this industry into the program,” said Bookout (oral communication, December
15, 2016). “Our goal is to get quality suppliers into the program, to
differentiate them from opportunistic suppliers, and to reduce the
ever-increasing cost of quality and qualifying suppliers.”
According to Bookout, the NASC’s program
has qualified most of the main ingredients commonly used in animal supplements
(see Table 1), such as glucosamine and chondroitin,* MSM,† and hyaluronic
acid.‡ However, the NASC hopes to start qualifying more herbal
ingredients (see Table 2) under the program soon.
According to data from the Chicago-based
market research firm SPINS, cross-channel aggregate** sales of animal
supplements totaled almost $110 million in the 52-week period that ended
November 27, 2016, an 11.2% increase in sales from the previous year. Table 1: Top-Selling Pet Supplements Overall
Primary Ingredient
| Sales
| % Change from Previous Year
| | Glucosamine | $21,244,413 | -6.1% | | Glucosamine/Chondroitin | $20,513,548
| 57.3% | | Vitamin E (not Ester-E) | $6,998,649
| 108.6% | | Multiple Vitamin (Adult) | $4,630,069
| -7.5% | | Animal Protein (Whey and Casein) | $3,934,666
| 127% | Parsley (Petroselinum crispum)
| $2,794,133
| -12.9% | | Zinc | $2,754,218
| 214.1% | | Animal and Plant Protein Combination | $2,544,139
| 19.5% | | Chlorophyll/Chlorella | $2,039,035
| -62.3% | Source: SPINS/Cross-channel aggregate** for the 52-week period ending November 27, 2017.
Table 2: Top-Selling Herbal Pet SupplementsPrimary Ingredient
| Sales
| % Change from Previous Year
| Parsley (Petroselinum crispum)
| $2,794,133
| -12.9% | | Chlorophyll/Chlorella | $2,039,035
| -62.3% | Mints (Mentha spp.)
| $1,831,908
| 38.2% | Pumpkin (Cucurbita pepo)
| $862,966
| 150.4% | Chamomile (Matricaria recutita)
| $465,978
| -32.6% | Flax Seed and/or Oil (Linum usitatissimum)
| $249,602
| 7.9% | Menthol (derived from Mentha species)
| $173,344
| -11% | Lavender (Lavandula angustifolia)
| $143,232
| -0.4% | Garlic (Allium sativum)
| $124,020
| -73.2% | Tea Tree Oil (Melaleuca alternifolia)
| $113,826
| 34.2% | Source: SPINS/Cross-channel aggregate** for the 52-week period ending November 27, 2017
The Preferred Supplier Program officially
began in 2014 and, in 2016, it grew significantly, according to Bookout. He
also said that the feedback the NASC has received about the program has all
been extremely positive.
Bookout said the NASC is also planning to
expand the program beyond dietary supplements for animals. “We are going to
expand the program out to pet food and pet treats manufacturers as well,” he
said.
The NASC’s Preferred Supplier Program was
put together with the help of NSF International auditors. “I have been told by
everyone who has looked at it that it will stand up to the independent scrutiny
of a third-party audit,” said Bookout. “At the end of the day, consumer
confidence is increased, and, in our case, the animals benefit.”
—Connor Yearsley
|
The
Regulatory Situation for the Animal Supplement Industry in the United States
The NASC was established to provide a
complete regulatory and compliance pathway for the animal supplement industry
because of the absence of a legal category for animal dietary supplements and
because of apparent efforts by regulatory bodies to remove many of these
products from the market.9
In 1994, the Dietary Supplement Health and
Education Act (DSHEA) was passed, and it classified dietary supplements as a
category of food under the Federal Food, Drug, and Cosmetic Act. This meant
that dietary ingredients could be used without premarket approval as long as
they were marketed in dietary supplements before October 15, 1994.9-11
However, DSHEA did not address animal
supplements, probably because that segment was very small at the time and
because the purpose of the legislation was to address the increasing demand for
human dietary supplements,9 as well as consumer and industry
concerns at the time about government regulations that would have limited
access to many supplements. In 1996, the Food and Drug Administration’s (FDA’s)
Center for Veterinary Medicine (CVM), which is primarily responsible for the
regulation of animal food and drugs, stated that DSHEA does not apply to animal
products, reasoning that ingredients with a history of safe use in human
dietary supplements may not necessarily be safe for animals. Different animal
species require different nutrients, they absorb and metabolize substances
differently, and they may exhibit different toxic reactions to substances.10
Therefore, products marketed as animal
dietary supplements, which typically fall under the category of “animal feed,”
are still subject to the pre-DSHEA regulatory environment and usually must be
made up of ingredients that are generally recognized as safe (GRAS), approved
as food additives, or listed in the Official
Publication of the Association of American Feed Control Officials.11
Animal supplements are not allowed to make
claims except about the nutrition, taste, and/or aroma of the product. In some
cases, the CVM allows claims of “nutritional support” of specific organs and/or
body functions. As with human dietary supplements, animal supplements that
claim to treat, mitigate, or prevent disease are considered unapproved new
drugs.11
The Food Safety Modernization Act (FSMA),
which was passed in 2011 and emphasizes preventing contamination in the food
chain instead of responding to it, established cGMPs for animal food
production. So, animal supplements marketed as food are subject to FSMA rules.12
|
* Glucosamine and chondroitin are compounds
derived from cartilage and are often used in combination to treat conditions
associated with osteoarthritis.6 † Methylsulfonylmethane
(MSM) is a natural compound found in some primitive plants (e.g., Equisetum arvense, Equisetaceae) and
other natural sources, and that can be prepared through oxidation of dimethyl
sulfoxide. It is used to treat a variety of conditions, such as arthritis,
joint inflammation, tendonitis, and musculoskeletal pain.7 ‡ Hyaluronic acid is a compound found in the
tissues and body fluids of vertebrates, and in some bacteria, that can help
with joint lubrication, water homeostasis, filtering effects, and regulation of
plasma protein distribution.8 ** Includes sales in the Natural Channel,
Specialty Gourmet Channel, and Conventional Channel. The SPINSscan Natural
Channel includes products sold at full-format natural product supermarkets,
small to mid-sized chains, and independent and cooperative stores across the
continental United States (excluding sales at Whole Foods Market). The SPINSscan Specialty Gourmet Channel includes products sold at full-format supermarkets with
more than $2 million in annual sales, with SPINS-defined specialty items making
up at least 25% of their overall volume. The SPINSscan Conventional Channel includes natural, organic,
specialty, and wellness products sold at conventional outlets in the United
States (data determined in collaboration with Information Resources Inc. [IRI
Worldwide]).
References
- Profile.
National Animal Supplement Council website. Available at: http://nasc.cc/profile/.
Accessed January 4, 2017.
- Frequently
Asked Questions. National Animal Supplement Council website. Available at: http://nasc.cc/faqs/.
Accessed January 4, 2017.
- NASC
Preferred Suppliers. National Animal Supplement Council website. Available at: http://nasc.cc/preferred-suppliers/.
Accessed January 4, 2017.
- Frequently
Asked Questions. SIDI Work Group website. Available at:
www.sidiworkgroup.com/FAQ. Accessed January 12, 2017.
- Guidance
for Industry: Current Good Manufacturing Practice in Manufacturing, Packaging,
Labeling, or Holding Operations for Dietary Supplements; Small Entity
Compliance Guide. Food and Drug Administration website. December 2010.
Available at: www.fda.gov/Food/GuidanceRegulation/GuidanceDocumentsRegulatoryInformation/DietarySupplements/ucm238182.htm.
Accessed January 4, 2017.
- Chondroitin.
University of Maryland Medical Center website. Available at: http://umm.edu/health/medical/altmed/supplement/chondroitin.
Accessed January 5, 2017.
- Methylsulfonylmethane
(MSM). Drugs.com website. Available at:
www.drugs.com/npp/methylsulfonylmethane-msm.html. Accessed January 12, 2017.
- Fraser
JRE, Laurent TC, Laurent UBG. Hyaluronan: its nature, distribution, functions,
and turnover. Journal of Internal
Medicine. 1997;242(1):27-33. doi: 10.1046/j.1365-2796.1997.00170.x.
- Regulation of Animal Health Supplements — A Historical Health Summary.
National Animal Supplement Council website. Available at: http://nasc.cc/historical-summary/.
Accessed January 6, 2017.
- Harrison
T, Jackson M. Pet Nutrition: A Legal Rundown. Nutraceuticals World website.
April 1, 2016. Available at:
www.nutraceuticalsworld.com/issues/2016-04/view_columns/pet-nutrition-a-legal-rundown.
Accessed January 6, 2017.
- DSHEA
Needed for Animal Supplements. Emord & Associates website. January 4, 2013.
Available at: http://emord.com/blawg/dshea-needed-for-animal-supplements/.
Accessed January 6, 2017.
- Final
Rule on Preventive Controls for Animal Food. Food and Drug Administration
website. Available at:
www.fda.gov/downloads/Food/GuidanceRegulation/FSMA/UCM461884.pdf. Accessed
January 6, 2017.
|