Issue:
105
Page: 34-45
Helichrysum italicum: The Sleeping Giant of Mediterranean Herbal Medicine
by Giovanni, PhD Appendino, Orazio Taglialatela-Scafati, PhD, Alberto Minassi,
PhD, Federica Pollastro, PhD, Laurea Mauro Ballero, Andrea Maxia, PhD, Cinzia Sanna,
PhD
HerbalGram.
2015; American Botanical Council
By Giovanni Appendino,Laureaa; Orazio Taglialatela-Scafati, PhDb;
Alberto Minassi, PhDa; Federica Pollastro, PhDa; Mauro
Ballero, Laureac; Andrea Maxia, PhDc; Cinzia Sanna, PhDc
aUniversità del Piemonte Orientale, Novara,
Italy
bUniversità di Napoli, Napoli, Italy
c Università di Cagliari, Cagliari, Italy
Introduction
Helichrysum italicum (Roth.) Don. (Asteraceae) is an iconic plant of the Mediterranean area (Figure 1), but the use of its essential oil in glamorous perfumes and personal care products has turned it into a veritable icon of luxury. However, just like the geographical distribution of Helichrysum species extends beyond the Mediterranean region, the properties of H. italicum are not limited to fragrance as they can benefit human health as well. In this context, H. italicum can be viewed as the sleeping giant of Mediterranean herbal medicine, and its extracts have the potential to be developed as dietary supplement ingredients just like its essential oil has been used successfully in perfumery and aromatherapy. Waking this giant will not be simple, but recent studies have provided the basis for a Helichrysum renaissance. This article outlines the fascinating ethnopharmacology of H. italicum in the light of modern molecular investigations of its constituents and their pharmacological targets, and highlights the extensive clinical studies carried out in 1930s Italy by Leonardo Santini, a physician and clinical investigator.

How Many Helichrysum Species Exist and Why is Their Color so Fade-Resistant?
The genus Helichrysum belongs to the family Asteraceae and encompasses more than 500 different species, with hotbeds of biodiversity centered in the Mediterranean basin, South Africa, and Australia — three geographically and geologically unrelated areas.1 This split distribution is presumably the result of parallel evolution from one or more ancestral and now-extinguished species. The name Helichrysum (from the Greek helios = sun and krysos = gold) makes reference to the bright yellow color of the flowerheads, which is particularly striking in full sun and also remarkably persistent in the dried plant. The name of the plant in many European languages — perpetuino and semprevivo in Italian, immortelle in French, everlasting in English, siempreviva in Spanish — recalls this property. The yellow color of Helichrysum’s flowerheads has been tied to the presence of the chalcone (a particular type of flavonoid) isosalipurposide,2 and its steadfastness may be due to stabilizing co-pigments, to the anatomy of the flower that preserves the bracts’ vacuolar contents from air oxidation, or to a combination of these factors. On the other hand, recent investigations of H. italicum failed to detect the presence of chalcones3 — which are probably typical of the Northern species H. arenarium Moench.4 — but found kaempferol-type flavonols instead.3 Therefore, the exact nature of the yellow pigment from H. italicum and, in general, of the resistance of Helichrysum flowerheads to fading, seem unclear. Interestingly, when mixed with mulberry (Morus spp., Moraceae) leaves and fed to silkworms, the flowerheads of H. italicum induce the production of naturally yellow silk, a material used in central Sardinia for the production of folk garments (Figure 2). Ancient Romans and Greeks decorated the statues of gods with wreaths of Helichrysum flowerheads, a practice mentioned by classic writers since the 7th century BCE. This use may seem curious, since existing classic statues are now the color of the original materials from which they were made (e.g., marble or bronze). However, they were originally vividly colored, and the yellow hue of Helichrysum in full sun would have given the effect of a gold crown on a polychromous figure.

Helichrysum italicum and its Baffling Scent
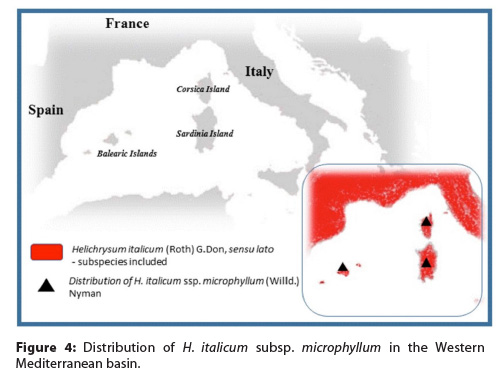
Helichrysum italicum is a xerophytic shrub 30 to 70 centimeters high, branched at the base with small, linear, hairy leaves that give the plant an overall grey hue until the appearance of the yellow flowerheads in June or July (Figure 3).1 It grows in dry, stony areas at an impressive range of altitudes from sea level to more than 2,000 meters. From a botanical standpoint, H. italicum is an umbrella name that covers at least six distinct varieties widespread mainly in the Western European Mediterranean region. The best-known variety grows around the northern Tyrrhenian Sea, on the islands of Corsica and Sardinia and on the Tuscany islands (Figure 4). The Tyrrhenian Helichrysum generally is referred to as H. italicum Don. subsp. microphyllum (Willd.) Nyman — a plant that also grows on the Balearic Islands off the eastern coast of Spain (e.g., Majorca and Minorca) — but recent taxonomical work classifies it as H. microphyllum subsp. tyrrhenicum Bacch., Brullo & Giusso.5 Tyrrhenian Helichrysum is the variety most valued for the production of an essential oil that is used in perfumery and personal-care products.6 Markedly, the Helichrysum note is used in commercial fragrances for both males and females (e.g., Magie Noire by Lancôme, Femme by Rochas, and Homme by Van Cleef & Arpels).
Helichrysum italicum is also the most-investigated species in terms of ethnobotany and phytochemistry. Six other species of Helichrysum are endemic in the Western Mediterranean area, including: H. frigidum (Labill.) Willd., H. montelinasanum E. Schmid, H. nebrodense Heldr., H. saxatile Moris, H. siculum (Sprengel) Boiss., and H. rupestre (Rafin.) DC.1 Each of these species has a distinct geographical distribution; H. montelinasanum, which grows only in a single location in Sardinia, has a particularly limited distribution.
Helichrysum italicum is famous and valued for its scent, which has been referred to as rosy, exotic, and spicy. The peculiar odor note was mentioned in the 1st century CE by Pliny the Elder, who, in his work Naturalis Historia, described it as not-at-all unpleasant and as being able to protect clothes from moths (Vestes tuetur odore non ineleganti).7 Indeed, the Tyrrhenian Helichrysum has a pleasant and persistent odor, different from that of the essential oil, and difficult to describe in terms other than “odor of Helichrysum.” It is reminiscent of the perfume of licorice (Glycyrrhiza glabra, Fabaceae), Indian curry, tobacco (Nicotiana tabacum, Solanaceae), and roses (Rosa spp., Rosaceae). Conversely, other European species of Helichrysum and other populations of H. italicum have an unpleasant phenolic note (which some people find annoying for its persistency)8* that is different from the manure-like note typical of some African Helichrysum species.9 Despite its significance, the odor issue has been poorly investigated in terms of both pleasant and unpleasant notes. During steam distillation, the licorice note is lost and a bitter, woody note is generated, presumably by the degradation of non-volatile constituents. Overall, the essential oil and the plant are quite different in terms of olfactory properties.

Ethnopharmacology of Helichrysum italicum
The largest body of ethnopharmacological information on H. italicum comes from the northern Tyrrhenian area, where the use of the plant is well documented in both human and veterinary medicine for the management of respiratory and digestive problems, and for topical wound healing.10-13 Thus, the plant is considered beneficial for inflammatory and infective airway conditions, including cough, bronchitis, laryngitis, and tracheitis. Helichrysum italicum also is used in herbal teas as a cholagogue (i.e., a bile stimulator) and choleretic agent. The so-called tisana del Quirinale (“Quirinale” is the Italian equivalent of the White House) is a combination of Helichrysum, lavender (Lavandula spp., Lamiaceae), and fennel (Foeniculum vulgare, Apiaceae), and the herbal tea was popular with two Presidents of Italy, Oscar Luigi Scalfaro and Carlo Azeglio Ciampi.14 Mixed with olive oil, H. italicum is used for the dermatological treatment of sun and fire burns and for wound healing. In veterinary medicine, the plant is used to treat cough in donkeys and joint ailments for both horses and donkeys.13 Curiously, the first modern investigations on the plant were spurred by these animal uses.
Helichrysum italicum also has culinary uses. These uses are quite different, however, from those of the archetypal edible Helichrysum (H. conchinchiensis), whose seedlings and sprouts are used in Asia to prepare banh khuc, a delicacy of Vietnamese cuisine. Helichrysum italicum is the source of one of the most expensive and highly sought-after European honeys, miele di spiaggia (seashore honey).15 Bees do not forage on H. italicum, but, while visiting the plant, they become covered with the resinous material that coats the flowers and pass it on to the pollen collected from other plants, eventually producing a honey that retains the flavor of Helichrysum. In the Tyrrhenian area, the leaves are used like rosemary (Rosmarinus officinalis, Lamiaceae) to flavor local dishes and salads, and to impart a “curry-like” note to pork meat and stuffings.16 The plant also is an ingredient in so-called Mediterranean curry (a mixture of H. italicum, onion [Allium cepa, Liliaceae], rosemary, wild fennel, thyme [Thymus vulgaris, Lamiaceae], and catnip [Nepeta cataria, Lamiaceae]). Owing to its anti-bacterial properties, H. italicum also has been used as a hop (Humulus lupulus, Cannabaceae) replacement in the production of beer. Finally, the use of Helichrysum to aromatize spirits and to prepare liqueurs is well documented, especially in Sardinia.
Helichrysum in the Classic Medical Treatises
Helichrysum is mentioned in all major medical treatises of the Greco-Roman tradition. In many of them, a botanical distinction is made between the species with large flowers (e.g., H. stoechas group, from the ancient name of the Hyères Islands off the French Côte d'Azur) and those with small flowers (e.g., H. italicum group) — the two main types growing in the Mediterranean area — but the same properties are assigned to both species. Theophrastus (3rd century BCE) — in his Historia Plantarum, the oldest extant medicinal plant treatise from the Western tradition† — mentioned the application of Helichrysum mixed with honey to treat burns and wounds,17 while Dioscorides (1st century CE) reported the use of a medicinal wine from Helichrysum to treat arthritic conditions and sciatica.19,20 Similar information is provided by Pliny the Elder in his Naturalis Historia, and the medical literature of the Renaissance often referred to these sources, with emphasis on the treatment of back and hip pain. The Italian Renaissance physician and botanist Castore Durante (1529-1590) also noted the use of a medicinal wine infused with H. italicum flowerheads to treat liver disorders, and further recommended a decoction of the plant for catarrh.21
Overall, the most common medical uses of Helichrysum documented by ancient authors were as a topical antiseptic, a cicatrizing (i.e., scar-forming) agent, for joint health and liver protection, and as a treatment for airway infections. Peculiarly, Theophrastus classified Helichrysum as a mind-altering plant,17 presumably because of its use in ritual fumigations. A similar use of Helichrysum is widespread in South Africa,9 and some species are traded as psychotropic agents.9 In fact, an African Helichrysum species is the only plant besides cannabis and hemp (Cannabis sativa, Cannabaceae) that contains cannabinoids, although researchers have isolated only the non-psychotropic agent cannabigerol (CBG) in the species.18
Helichrysum Essential Oil and its Use in Medicine and Aromatherapy
The essential oil of Helichrysum is obtained from biomass of various geographical origins (e.g., the Balkan Peninsula, Ukraine, Corsica, Sardinia, Provence, and Spain),6 and once was used mainly for the aromatization of pipe tobacco. Factors related to the Balkan wars of the 1990s as well as Helichrysum’s increased popularity in aromatherapy, perfumery, and cosmetics, led to a shortage of plant material that stimulated cultivation and collection in the Western part of the Mediterranean region, especially in Corsica and Sardinia.6 The absolute is used only in perfumery, while the essential oil is employed in medicine and aromatherapy. The introduction of the oil of H. italicum in aromatherapy originated in France, and was popularized in English-speaking countries by aromatherapist and author Kurt Schnaubelt, who notably considers interchangeable the many uses of the pinene- and neryl ester-rich oils.
The essential oil of Tyrrhenian Helichrysum is significantly different from the oil of H. italicum subspecies and other Helichrysum species; it is characterized by a high content (25-45%) of neryl esters (acetate, isobutyrate, isovalerate), which are considered the plant’s most distinctive constituents.22-26 Also, the sesquiterpenoid fraction of the oil (about 10-20%) is distinctive, containing the bisabolane-deriviative curcumene, bisabolane cycloadducts (italicenes), and eudesmane alcohols (rosifoliol).22-26 The oils of Helichrysum from the Balkan peninsula and the Adriatic coast of Italy contain mainly pinenes, and, like some oils of French cultivation, they have a high curcumene content (ca. 20%).6 The oils of Helichrysum also contain a series of diketones (italidiones) probably derived from the thermal degradation of non-volatile pyrones that are abundant constituents of several Helichrysum species.22-26 Since these diketones are likely artifacts, their concentrations may be related to the duration of the distillation process, but their value as markers is limited because of their presence in the oils of other species of Helichrysium. The licorice note of Tyrrhenian Helichrysum is lost during steam distillation, while the compound(s) responsible for the unpleasant phenolic odor of some Helichrysum plants and oils, as previously mentioned, have not been characterized yet. Despite the popularity of Helichrysum essential oil, the molecular bases of its sensory properties have not been characterized fully.
Helichrysum oil is obtained by steam distillation of the flowerheads, and its yield is rather low, rarely above 0.1% of the starting fresh biomass.22-26 This, along with the low biomass production of the plant, is responsible for the high price of the oil, which is currently one of the most expensive on the market (roughly 2,000 euro/kg). The composition of the oil is variable, even among samples of the same geographical origin; the texture and acidity of the soil seem to have a marked effect. Rich in neryl esters and diketones, Tyrrhenian Helichrysum oil is the variety most valued in perfumery and most used in aromatherapy, although it is not clear if the contents of neryl esters and diketones alone can explain this preference.6,26 Owing to differences in the composition of the essential oil from H. italicum, it is possible that specific properties are associated with specific types of essential oil, but this issue has not yet been systematically investigated.
The main use of the essential oil of Helichrysum is to fade or reduce scars. Despite the lack of animal and human studies to substantiate this application, many practitioners are convinced of its utility for this purpose.27 Another important use of the oil is for the treatment of bleeding wounds, an indication for which it is considered the essential oil of preference. For this indication, it has been suggested that the oil is best used undiluted to help sanitize the wound, foster its closure, and relieve pain. For all other uses, the oil is diluted in fixed oil.27 The essential oil also has been used in combination with lavender (Lavandula angustifolia, Lamiaceae) and tea tree oil (Melaleuca alternifolia, Myrtaceae) to soothe skin conditions associated with chemotherapy such as palmar-plantar erythrodysesthesia, otherwise known as hand-food syndrome (a trophic skin disorder associated with the use of anticancer drugs, including both cytotoxic [capecitabine, taxanes, camptothecins] and targeted [monoclonal antibodies such as cetuzimab as well as kinase inhibitors including erlotinib, sorafenib, and sunitinib] chemotherapy agents).27 Similar combinations of essential oils with anti-VEGF (vascular endothelial growth factor) or anti-EGF (epidermal growth factor) agents can be used to manage chemotherapy-induced acne and the “face powder” desquamation associated with the use of 5-fluorouracile and irinotecan.27 Despite the success of Helichrysum oil as an anti-aging agent, no published clinical data support the “Fountain of Youth” claims of some cosmetic products, although proprietary data might exist.
The growing use of Helichrysum oil in aromatherapy and medicine should stimulate randomized, controlled trials to assess its efficacy and help define standards of composition for the medicinal use of the oil, as has been done with neryl esters for use in perfumery.
Modern Studies on Helichrysum: The Contribution of Leonardo Santini
Leonardo Santini (Figure 5) is considered the father of modern studies on Helichrysum. He was born in 1904 in Molazzana, a small village near Lucca, in Northern Tuscany.‡ His father was a physician, as were many of his relatives. Santini earned a degree in medicine from the University of Pisa and, later, started a medical practice in Castelnuovo di Garfagnana, a small town in Northern Tuscany famous for being governed during the Renaissance by the poet Lodovico Ariosto. The involvement of Santini with Helichrysum is reminiscent of that of William Withering with foxglove (Digitalis purpurea, Plantaginaceae). Just like Withering was “tipped” on the properties of foxglove by an “old lady” of a country village, Santini was introduced to Helichrysum’s healing properties by a mule-driver who claimed to have successfully managed his donkeys’ cough with canugioro, the local name for H. italicum. (Since donkeys are more stoic than horses, cough generally is indicative of an advanced respiratory disease.) This, coupled with his awareness of the plant’s widespread use for similar conditions in horses, led Santini to administer a decoction of the plant to some of his patients who were suffering from bronchitis and asthmatic cough. Not only did their respiratory conditions improve, but also other unrelated diseases from which they were suffering — particularly psoriasis and arthritis. Intrigued by these observations, Santini started to experiment with a decoction and syrup from H. italicum in the late 1930s, summarizing more than two decades of observations in a series of articles that were published from 1949 to 1953 in Italian journals with unfortunately limited distribution outside the community of general practitioners.28-31 Santini determined that the clinical activity of Helichrysum was similar of that of cortisone — a drug in short supply at the time — and also reported an insulin-sensitizing activity in animal experiments. The proposed cortisone-like compound was named helichrysin, but it was never characterized chemically. Santini found the decoction’s anti-psoriatic activity to be particularly useful and treated hundreds of patients with the condition. The beneficial effects of the treatment were confirmed in two independent clinical studies carried out in the 1950s.28-31 More recently, the results of a small open study from 1995 supported the use of a 5% decoction for the treatment of psoriasis.32 After three weeks of treatment, all study participants improved, with relapses observed within two months post-treatment. Though spontaneous regression of psoriasias is not uncommon, these studies provide a rationale for the initiation of controlled, double-blind, clinical trials for the use of H. italicum in managing psoriasis.

Santini also observed positive results from the use of an aerosolized Helichrysum decoction for allergic rhinitis and a Helichrysum ointment for the treatment of solar and fire burns, chilblains, venous stasis, varicose veins, and hemorrhoids. Interestingly, a topical product containing an extract from H. arenarium (L.) Moench. was developed in the former USSR for the management of radiation burns.33
The publication of Santini’s studies in the early 1950s piqued the interest of Renzo Benigni — the chief pharmacologist of the Italian company Inverni della Beffa — who started systematic studies on the plant, including the formulation used by Santini (a simple 5% decoction of the dried flowerheads). Benigni found that filtration of the decoction led to a considerable loss of activity, suggesting that the active constituents had limited solubility in water, where it was dispersed and not well dissolved. To obtain more reproducible animal data, he investigated an extract (“Fraction H”) produced using an organic solvent. This product exhibited some cortisone-like activity, and, at a dosage of 5 mg/day, could significantly prolong the life of rats whose adrenal glands had been surgically removed.33 A series of clinical studies in various Italian centers substantially confirmed the findings of Santini, showing that Fraction H could replace, to varying degrees, corticosteroids in many of their uses without their adverse side effects.33 Benigni, however, never published his findings; he only summarized them in a Helichrysum monograph that appeared in the monumental treatise on medicinal plants he coauthored with Capra and Cattorini.33 In the wake of Santini’s studies, a Helichrysum syrup was commercialized in the Italian pharmaceutical market as a cough syrup. However, the lack of standardization and information on the active principle led to the product’s demise when more stringent rules on pharmaceutical products were introduced in Italy in the late 1960s. Notably, a small clinical study in 1981 on children affected by tracheo-bronchitis found a Helichrysum decoction highly efficacious.34
Phytochemical and Pharmacological Studies on Helichrysum italicum
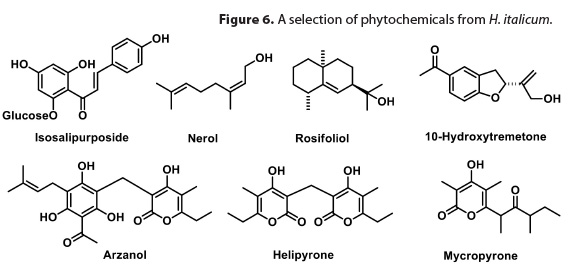
Most recent phytochemical analyses of H. italicum have focused on the northern Tyrrhenian variety (H. italicum subps. microphyllum), which Santini used in his clinical studies. The most common non-volatile constituents of this plant belong to the acylphloroglucinol and pyrone classes of ketides. These compounds can occur in H. italicum as a monomer (micropyrone), symmetrical dimer (helipyrone), or mixed dimer (arzanol) (Figure 6).35
Arzanol is the best
characterized of these ketides in terms of bioactivity. Its name is derived
from the small town of Arzana (Figures 7) in East Sardinia, where the plant
that yielded this compound was collected.35 Arzanol
has an anti-inflammatory profile not unlike that of curcumin, suggesting that
the association of H. italicum with curry might extend beyond olfaction.
(Both turmeric and Helichrysum
essential oils contain the volatile curcumene sesquiterpene.) Arzanol
interrupts pro-inflammatory signaling and increases antioxidant protection by
acting at distinct time-domain targets.36 The enzymes mPGES-1 and
5-LO are key players in the generation of inflammatory eicosanoids (the
prostaglandin PGE2 and leukotrienes, respectively) and represent the
short-time domain target for arzanol, while the pro-inflammatory transcription
factors NF-κB represent its primary long-term target.35 The
net result is the inhibition of the production of inflammatory cytokines (TNF-α,
IL-1b, IL-6) and inducible inflammatory enzymes (COX2 and mPG2S). A study in
2011 found that, in contrast with non-steroidal anti-inflammatory drugs
(NSAIDs), the production of beneficial prostanoids like PI2 was not
affected by arzanol, which retained activity in an in vivo model of
inflammation (carrageenan-induced pleurisy in rats).36

Arzanol is refractory to chemical modification, but its structure-activity relationships can be elucidated by total synthesis, highlighting the relevance of its decoration of hydroxyls and heterodimeric structure for the inhibition of mPGES-1 and 5LO.37 The symmetrical pyrone dimer helipyrone was much less potent against these anti-inflammatory targets, as was the monomeric phloroglucinol micropyrone. Arzanol also has shown remarkable antibacterial activity, outperforming the fluoroquinolone norfloxacin against many strains of super-bacteria of the infamous methicillin-resistant staphylococcus aureus (MRSA) type.38 Polar fractions from extracts of H. italicum display anti-biofilm activity against the gram-negative pathogen Pseudomonas aeruginosa.39 In this context, it is interesting to note that a mixture of heterodimeric phloroglucinols known as arenarin was developed as a skin-and-eye antibacterial agent in the former Soviet Union.40 The polyphenolic phloroglucinyl component makes arzanol a potent antioxidant, equipotent to caffeic acid and more potent than other plant-derived polyphenolics such as nordihydroguaiaretic (NDGA) acid, magnolol, and even myrtucommulone from myrtle (Myrtus communis, Myrtaceae) in in vitro assays of antioxidant activity.37,41 Arzanol also was shown to inhibit lipid peroxidation in a series of in vivo systems.42 Taken together, these observations suggest that arzanol may be the major bioactive constituent of helichrysin, the crude preparation of Helichrysum used by Santini in his clinical investigations.
Apart from arzanol, H. italicum contains additional bioactive constituents that could complement its anti-inflammatory activity. For instance, the flowerheads and aerial parts are rich sources of ursolic acid (up to 0.5% by dry weight)38 — an anti-inflammatory triterpenoid and popular cosmetic ingredient43 — and also of benzofurans of the tremetone type,38 some of which have shown anti-spasmodic effects.44 A mixture of methylketones and sterols known as tremetol was long considered the toxic agent responsible for the plant-induced “milk sickness” that devastated large areas of the American Midwest in the first half of the 19th century (and whose most famous victim was Abraham Lincoln’s mother).45 However, pure synthetic tremetone was not capable of reproducing the toxicity of extracts from white snakeroot (Ageratina altissima, Asteraceae), which is now considered the most likely plant source of milk sickness, although the nature of the toxin(s) involved is still unclear.46 Also noteworthy is the presence of an unusual class of lipids, named santinols in honor of Leonardo Santini, in Tyrrhenian H. italicum.38 Santinols are glycerides of branched medium-chain acids that represent a novel type of plant lipids. Curiously, one of the acids present in santinols (2-methyl-3-oxovaleric acid) is a marker for the diagnosis of propionic acidemia, a human genetic disease, and had never been identified before in plants.38
Helichrysum italicum shows remarkable plasticity in terms of growing conditions, thriving both on the coast and in the mountains with no apparent preference for a particular type of substrate (calcareous, acidic, sandy, stony). This ecological adaptability translates into a heterogeneous morphology, the presence of numerous ecotypes and varieties, and, ultimately, phytochemical polymorphisms. Thus, several chemotypes of H. italicum exist, characterized by distinct phytochemical profiles and therefore unlikely to be pharmacologically equivalent. In particular, the presence of high concentrations of arzanol seems characteristic of the northern Tyrrhenian population of H. italicum (subps. microphyllum); commercial samples from the southern Tyrrhenian area as well as those of Adriatic origin did not contain significant amounts of this compound.47
The Helichrysum italicum Supply Chain
Helichrysum species have been commercially available as ornamentals since the 17th century. The species popular in nurseries today are mainly non-European, including H. bracteatum Andr. from Australia, H. bellidioides Willd from New Zealand, and H. petiolatum DC from South Africa. The most popular European species available in nurseries are H. stoechas (L.) Moench. from the Balkans and H. arenarium (L.) Moench. DC. from Eastern Europe. Helichrysum italicum is of more limited availability in the horticultural market; the commercial material used by the perfumery and cosmetic industries is rather heterogeneous and is derived from both cultivation and wild collection in different regions.6 Apart from the northern Tyrrhenian area, H. italicum can be found along the Adriatic coast of Italy, the estuary of the river Po, and in Southern Italy. The major areas of H. italicum cultivation in the Western Mediterranean region are Corsica and Sardinia, and most biomass produced there is acquired by the cosmetic and perfumery industries and distilled. Essential oil production is highly fractionated, and it is difficult to estimate the total amount of oil produced. The medicinal supply chain of H. italicum remains underdeveloped, and, given the high price of the essential oil, many producers find it more convenient to distill the plant and sell the oil rather than to commercialize the whole plant for the production of extracts. The essential oil is produced exclusively from the flowerheads, while the rest of the plant — which contains significant amounts of secondary metabolites and could in principle be used to produce extracts — is left in situ.48
The expense of the oil, especially of Tyrrhenian origin, provides incentive for economically motivated (i.e., intentional) adulteration. Currently, there are no established reference criteria for medicinal-grade Helichrysum oil, but the aromatherapy market, like the perfumery market, emphasizes oil of Corsican origin, which sells at a considerably higher price. Remarkably, some vendors of “Corsican” Helichrysum oil provide gas chromatograph profiles on their websites that do not qualify the oil as of Corsican or Tyrrhenian origin. Low nerol contents and high hydrocarbon contents — like the Balkan and Adriatic Helichrysum oils — indicate either adulteration or the cultivation in Corsica of non-native chemotypes of foreign origin.49 The limited production of Tyrrhenian Helichrysum oil and its relevance in the perfumery and personal care industries call into question whether or not significant amounts of the oil are in fact available on other markets.
Today, there is a huge disconnect between demand and availability of Tyrrhenian Helichrysum, which has been compounded by the past years’ poor harvest. Adulteration of some materials is filling this gap now, but wild harvesting is a growing problem in both Corsica and Sardinia, despite the hard-to-reach location of many populations of the plant. (Sardinia and Corsica have the longest undeveloped coastal areas in Western Mediterranean Europe.) Wild harvesting will not only significantly impact the status of the plant, but, by providing a lower-cost (but unsustainable) plant material, it also is damaging the ongoing projects intended to develop a sustainable Helichrysum supply chain and meet its strongly growing demand. Due to its superior properties, the Sardinian population of gentian (Gentiana lutea, Gentianaceae) was exterminated in the 1950s and 1960s by uncontrolled harvesting, and, despite efforts of re-introduction, it has not recovered. To avoid a similar fate for Helichrysum, a program to certify the plants’ sustainable cultivation through to harvest should be established for the plant, which also would foster consumer awareness of the fragility of the supply chain from wild harvesting.
Conclusions
Extracts from H. italicum are used in topical products for the management of hemorrhoids and lower limb venous insufficiency (i.e., heavy legs) in the Italian healthfood market. These extracts are not standardized in the phytochemicals typical of H. italicum, and these uses do not represent the full potential of the plant. Santini published the results of his investigations on the presence of a cortisone-like compound in H. italicum in the 1940s, when the first synthetic corticosteroids were developed. The availability of corticosteroids — well characterized chemically and relatively low cost — undermined interest in Santini’s findings. Owing to the progress in chemistry, from the 1930s onward plant extracts had been increasingly replaced by their single primary active ingredients (lanatosides for foxglove, atropine for nightshade [Solanaceae]) or synthetic analogs (amphetamine for ephedrine). Meanwhile, the work of Santini was going in the other direction, apparently against the flow of medical history. The lack of information on the active ingredients of Helichrysum, the complications involved in the standardization of a phytochemically unexplored plant, and the isolation of Santini — who was not part of the medical establishment, self-financing research on helichrysin, and publishing his results in medical journals of limited distribution — led to a loss of interest in H. italicum and its cortisone-like properties. In the past decade, the discovery of arzanol, a compound with pleiotropic (multiple effects emanating from one single compound) anti-inflammatory activity, and a better phytochemical characterization of the plant have set the stage for the development of standardized preparations of H. italicum. A systematic investigation of their clinical potential will reposition H. italicum from the glittering world of perfumes and fashion to the world of medicine, where it also belongs.
Giovanni Appendino, Laurea, is a full professor of organic chemistry at the Universita del Piemonte Orientale in Novara, Italy. Also at the Universita del Piemonte Orientale, Alberto Minassi, PhD, is a lecturer of organic chemistry, and Federica Pollastro, PhD, is a lecturer of pharmacognosy.
Orazio Taglialatela-Scafati, PhD, is an associate professor of organic chemistry at the Università di Napoli in Napoli, Italy.
Mauro Ballero, Laurea, and Andrea Maxia, PhD, are associate professors of pharmacognosy, and Cinzia Sanna, PhD, is a lecturer of pharmacognosy at the Università di Cagliari in Cagliari, Italy.
*The foul odor is mentioned in some books as a distinctive character of H. italicum (see, for instance: Riva E. Piante Medicinali. Ghedini e Tassotti Editori; 1995, p. 274.
† For a superbly annotated modern edition of Theophrasto’s Historia Plantarum, see: Amigues S. Théophraste Recherches sur le plantes. Belin, Paris, 2010. For a survey on the anti-inflammatory plants mentioned in herbaria from the XVI-XVII century, see: Adam M, Berset C, Kessler M, Hamburger M. Medicinal herbs for the treatment of rheumatic disorders—A survey of European herbals from the 16th and 17th century. J Ethnopharmacol 2009;121:343-359.
‡ Most information on the life of Leonardo Santini, including his picture, was provided by his son Maurizio, to whom the authors are grateful. The life of Leonardo Santini also was outlined in a commemorative speech given by Paolo Mantegazza, the Rector of the University of Milano and the former Dean of the School of Medicine of Milano, on May 9, 2004 at Castelnuovo di Garfagnana during the commemoration of the 100th anniversary of the birth of Leonardo Santini.
§ For a review on the biological profile of arzanol, see: Kothavade, PS, Nagmoti DM, Bulani VD, Jvekar AR. Arzanol, a potent mPGES-1 inhibitor: novel anti-inflammatory agent. Scient World J. 2013; doi: 10.1155/2013/986429.
References
- Anderberg AA. Taxonomy and phylogeny of the tribe Gnaphalieae (Asteraceae). Opera Bot. 1991;104:1-195.
- Kurkina AV, Ryzhov VM, Avdeeva EV. Assay of isosalipurposide in raw material and drugs from the dwarf everlast (Helichrysum arenarium). Pharm Chem J. 2012;46:171-176.
- Mari S, Napolitano A, Masullo M, Pizza C, Piacente S. Identification and quantitative determination of the polar constituentsin Helichrysum italicum flowers and derived food supplements. J Pharm Biomed Anal. 2014;96:249-255.
- Morikawa T, Wang LB, Nakamura S, Ninomiyta K, Yokoyama E, Matsuda H, Muraoka O, Wu LJ, Yoshikawa M. New flavanone and chalcone glycosides, arenariumosides I, II, III, and IV, and tumor necrosis factor-ainhibitors from everlasting, flowers of Helichrysum arenarium. Chem Pharm Bull. 2009; 57:361-367.
- Angiolini C, Bacchetta G, Brullo S, Casti M, Giusso del Galdo G, Guarino R. The vegetation of mining dumps in SW-Sardinia. Feddes Repertorium. 2005;116:243-276.
- Hellivan PJ. Immortelle sustainable resurgence. Perfumer & Flavorist. 2009;34:34-40.
- Pliny the Elder. Naturalis Historia. Liber XXI: 168-169.
- Cattorini PE. Fitoterapia. 1949;23:51-56.
- Helichrysum odoratissmum – Imphepho. Entheology website. Available at: http://entheology.com/plants/helichrysum-odoratissmum-imphepho/. Accessed November 18, 2014.
- Antunes Viegas D, Palmeira-de-Oliveira A, Salgueiro L, Martinez-de-Oliveira J, Palmeira-de-Oliveira R. Helichrysum italicum: From traditional use to scientific data. J Ethnopharmacol. 2014;151:54-65.
- Ballero M, Poli F, Sacchetti G, Loi MC. Ethnobotanical research in the territory of Fluminimaggiore (south western Sardinia). Fitoterapia. 2001;72:788-780.
- Bruni A, Ballero M, Poli F. Quantitative ethnopharmacological study of the Campidano Valley and Urzulei district, Sardinia, Italy. J Ethnopharmacol. 1997;57:97-124.
- Uncini Manganelli RE, Tomei PE. Ethnopharmacobotanical studies of the Tuscan Archipelago. J Ethnopharmacol. 1999;65:181-202.
- Tisana de Quirinale. Available: www.dottoressacagnola.it/tisam/quirinale.html. Accessed November 18, 2014.
- Beach Honey. Arte Toscana website. Available at: www.artetoscana.it/miele/533-miele-di-spiaggia-biologico.html. Accessed November 18, 2014.
- Ghirardini MP, Carli M, del Vecchio N, Rovati A, Cova O, Valigi F, et al. The importance of a taste. A comparative study on wild food plant consumption in twenty-one local communities in Italy. J Ethnobiol Ethnomed. 2007;4:3:22.
- Theophrastus. Historia Plantarum. Liber IX: 19.
- Bohlmann F, Hoffmann E. Cannabigerol-aehnliche Verbindunged aus Helichrysum umbraculigerum. Phytochemistry. 1979; 18:1371-1374.
- Dioscorides. Materia Medica. 4:57.
- Mattioli Pietro Andrea. I Discorsi ne i Sei Libri di Pedacio Dioscoride. Venezia; 1544: 534.
- Durante Castore. Herbario nuovo. Roma; 1585: 222.
- Bianchini A, Tomi P, Costa J, Bernardini AF. Composition of Helichrysum italicum (Roth) G. Don fil. subsp italicum essential oils from Corsica (France). Flavour & Fragr J. 2001;16:30-34.
- Angioni A, Barra A, Arlorio M, Coisson JD, Russo MT. Pirisi FM, Satta M, Cabras P. Chemical composition, plant genetic differences, and antifungal activity of the essential oil of Helichrysum italicum G. Don ssp. microphyllum (Willd) Nym. J Agric Food Chem. 2003;51:1030-1034.
- Morone-Fortunato I, Montemurro C, Ruta C, Perrini R, Sabetta W, Blanco A, et al. Essential oils, genetic relationships and in vitro establishment of Helichrysum italicum (Roth) G.Don ssp. italicum from wild Mediterranean germplasm. Ind Crops Prod. 2010;32:639-649.
- Leonardi M, Ambryszewska KE, Melai B, Flamini G, Cioni PL, Parri F, et al. Essential-oil composition of Helichrysum italicum (ROTH) G.DON ssp. italicum from Elba Island (Tuscany, Italy). Chem Biodivers. 2013;10:343-55.
- Melito S, Sias A, Petretto GL, Chessa M, Pintore G, Porceddu A. Genetic and metabolite diversity of Sardinian populations of H. italicum. PLoS One. 2013;18;8(11):e79043.
- Schnaubelt K. The Healing Intelligence of Essential Oils. Healing Art Press, Toronto; 2011: 152-155.20.
- Santini L. Considerazioni sugli effetti terapeutici dell’Elicriso, ed. Tipografia Salvietti, Castelnuovo Garfagnana. 1948.
- Santini L. Atti Soc Lomb Sci Med Biol. 1949;5:18.
- Santini L. Riv. di Terapia Pratica n. 169, gennaio-febbraio 1949.
- Santini L. Rassegna clinico-statistica sulle proprietàterapeutiche dellélicrisio (Helichrysum italicum). Minerva Med. 1952;43:714-719.
- Campanini E. Helichrysum angustifolium: Esperienze cliniche sulla psoriasi. Acta Phytotherapeutica. 1995;1:8-10.
- Benigni R, Capra C, Cattorini PE. Piante Medicinali. Chimica, Farmacologia e Terapia. Milano; 1962:533-547.
- Vannini C. Atti II Seminario Internazionale Piante Medicinali e Medicina Tradizionale. Città di Castello, 1981. Quoted in ref. 32.
- Appendino G, Ottino M, Marquez N, Bianchi F, Giana A, Ballero M, et al. Arzanol, an anti-inflammatory and anti HIV-1 phloroglucinol alpha-pyrone from Helichrysum italicum ssp microphyllum. J Nat Prod. 2007;70:608-612.
- Bauer J, Koeberle A, Dehm F, Pollastro F, Appendino G, Northoff H, et al. Arzanol, a prenylated heterodimeric phloroglucinyl pyrone, inhibits eicosanoid biosynthesis and exhibits anti-inflammatory efficacy in vivo. Biochem Pharmacol. 2011;81:259-268.
- Minassi A, Cicione L, Koeberle A, Bauer J, Laufer S, Werz O, Appendino G. A multicomponent carba-Betti strategy to alkylidene heterodimers – Total synthesis and structure-activity relationships of arzanol. Eur J Org Chem. 2012:772-779.
- Taglialatela-Scafati O, Pollastro F, Chianese G, Minassi A, Gibbons S, Arunotayanun W, et al. Antimicrobial phenolics and unusual glycerides from Helichrysum italicum subsp. microphyllum. J Nat Prod. 2013;76:346-353.
- D’Abrosca B, Buommino E, D’Angelo G, Coretti L, Scognamiglio M, Severino V, et al. Spectroscopic identification and anti-biofilm properties of polar metabolites from the medicinal plant Helichrysum italicum against Pseudomonas aeruginosa. Bioorg Med Chem. 2013;21:7038-7046.
- Bel’yukova, K. G. Plant antibiotic, arenarin. Mikrobiologichnii Zhurnal.1968;30:390-398.
- Rosa A, Deiana M, Atzeri A, Corona G, Incani A, Melis MP, et al. Evaluation of the antioxidant and cytotoxic activity of arzanol, a prenylated alpha-pyrone-phloroglucinol etherodimer from Helichrysum italicum subsp. microphyllum. Chem Biol Interact. 2007;165:117-126.
- Rosa A, Pollastro F, Atzeri A, Appendino G, Melis MP, Deiana M, Incani A, Loru D, Dessì MA. Protective role of arzanol against lipid peroxidation in biological systems. Chem Phys Lipids. 2011;164:24-32.
- Ikeda Y, Murakami A, Ohigashi H. Ursolic acid: an anti- and pro-inflammatory triterpenoid. Mol Nutr Food Res. 2008;52:26-42.
- Rigano D, Formisano C, Senatore F, Piacente S, Pagano E, Capasso R, et al. Intestinal antispasmodic effects of Helichrysum italicum (Roth) Don ssp. italicum and chemical identification of the active ingredients. J Ethnopharmacol. 2013;150:901-906.
- Couch, JF. The toxic constituent of richweed or white snakeroot (Eupatorium urticaefolium). J Agric Res. 1927; 35:547-576.
- Lee ST, Davis TZ, Gardner DR, Steglemeier BL, Evans TJ. Quantitative method for the measurement of three benzofuran ketones in rayless goldenrod (Isocoma pluriflora) and white snakeroot (Ageratina altissima) by high-performance liquid chromatography (HPLC). J Agric Food Chem. 2009; 57:5639-5643.
- Appendino G: unpublished data.
- Elicriso VG. Veneto Agricoltura. Piante officinali. 2001;1:1-12.
- Data file. Helychrisum italicum website. Available at: www.helichrysum-italicum.com/helichrysumMarch2014Analysis.pdf. Accessed on December 16, 2014.