Issue:
106
Page: 56-58
Botanical Integrity: The Importance of the Integration of Chemical, Biological, and Botanical Analyses, and the Role of DNA Barcoding
by Charlotte Simmler, PhD, Shao-Nong Chen, PhD, Jeff Anderson, MS, David C. Lankin, PhD, Rasika Phansalkar, Elizabeth Krause, PharmD, Birgit Dietz, PhD, Judy L. Bolton, PhD, Dejan Nikolic, PhD, Richard B. van Breeman, PhD, Guido F. Pauli, PhD
HerbalGram.
2015; American Botanical Council
Editor’s note: This article was originally published in the April 2015 issue of HerbalEGram.
What are Botanicals?
Raw materials, ingredients, and products derived from plants are commonly referred to as herbs or botanicals in both the biomedical literature and the natural products health industry. This overarching term includes the breadth of crude herbs, plant parts, and the ingredients made from them, and also covers finished products such as botanical dietary supplements. Botanical dietary supplements are intended to supplement the human diet and are comprised primarily of powdered plant parts, their extracts, or other preparations derived from crude herbal material; some formulations include other ingredients such as vitamins, minerals, and amino acids. Botanical dietary supplements are highly complex mixtures reflecting the diverse chemical constituents that compose the source plant’s raw material. Botanical analysis is an intricate analytical challenge requiring specialized skills and instrumentation that is different from those required for quality control of chemically simpler pharmaceuticals, or for the safety assessment of many conventional foods or other products that are generally recognized as safe (GRAS).
 What is Botanical Integrity?
The concept of Botanical Integrity evolved from an initiative by the National Center for Complementary and Integrative Health (NCCIH, formerly the National Center for Complementary and Alternative Medicine [NCCAM]) and the Office of Dietary Supplements (ODS), both at the US National Institutes of Health (NIH). The initiative led to the implementation of the NIH natural products integrity policy,1 which addresses botanical study materials by outlining special requirements for their characterization. Going beyond just “quality control,” Botanical Integrity combines disparate aspects of defining and assessing plant-derived materials and products for human consumption: identity (correct plant species and plant part), homogeneity (absence of contamination by other species and chemicals; often called “purity” but different than the regulatory use of that term), biological potency (the presence of bioactive principles in desired amounts; a prerequisite for in vivo efficacy), and safety (an adequate toxicological profile).
How can Botanical Integrity be assessed?
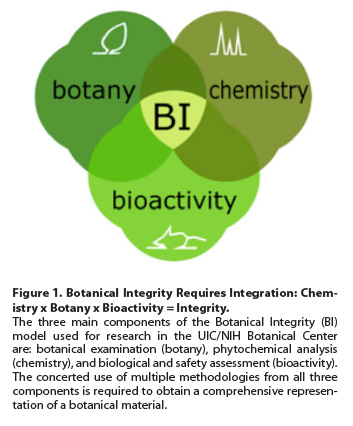
The assessment of Botanical Integrity requires a multidisciplinary approach that combines three major domains of expertise, with each providing a variety of tools and analytical methodologies (See Fig. 1): botanical examination (botany), phytochemical analysis (chemistry), and biological and safety assessment (bioactivity). Botanical examination is performed on the source material and involves operations typically performed in plant taxonomy, morphology, and genetics. Phytochemical analysis can be carried out with crude plant material, extracts, and finished products. These analyses encompass a wide array of chemical analytical tests with major domains in chromatography (e.g., liquid chromatography [LC], gas chromatography [GC], thin-layer chromatography [TLC]) and spectroscopy/spectrometry (e.g., ultraviolet [UV], infrared [IR], near-infrared [NIR], mass spectrometry [MS], nuclear magnetic resonance [NMR]). Operations for the assessment of bioactivity and safety, which are typically performed with botanical extracts, comprise in vitro and sometimes in vivo assays for endpoints related to the health benefit and/or safety of the herb. Investigations in the UIC/NIH Center for Botanical Dietary Supplements Research have integrated various aspects of all three of these fundamental domains (botany, chemistry, and bioactivity) since 1999. The UIC/NIH Center has pioneered new methodologies for the assessment of Botanical Integrity and produced insights into the safety and efficacy of botanical dietary supplements that are widely used for women’s health. We have also implemented Botanical Integrity Dossiers as a means of documenting comprehensively the Botanical Integrity of our study materials, including crude herbal material, extracts, and purified phytochemicals.
How does Botanical Integrity help ensure safety and efficacy?
Under the governance of the Dietary Supplement Health and Education Act of 1994 (DSHEA), botanical products may be marketed as dietary supplements as long as they meet the appropriate regulatory requirements within that category of food, not drug, regulations. Category-specific current Good Manufacturing Practices (cGMP) and labeling requirements are in place for botanical dietary supplement manufacturers and have fostered the development of a marketplace with a broad range of high-integrity products. However, adulteration and other issues affecting the Botanical Integrity of products can still occur, and the combination of botanical, chemical, and biological standardization is a viable approach for ensuring safe and effective botanical products.
What is DNA barcoding?
The authentication of botanical raw materials (unextracted fresh and dried plant parts) has benefitted from the implementation of a variety of molecular biology methods based on the analysis of DNA sequences, and it was originally employed for plant systematics (i.e., the classification and naming of plants).2-4 Among these methods, DNA barcoding relies on the amplification and sequencing of short (400-800 base pairs) nucleotide sequences localized to standardized regions of the genome (plastid and nuclear loci for plants). Species are identified according to the nucleotide variations in these short standardized gene regions, which give them unique DNA identification tags.2,3,5 During the last decade, DNA barcoding has emerged as a method for the authentication of plant parts and herbal materials.6-10
Recently, a DNA-based authentication method was employed to evaluate the identities of 78 samples from commercial botanical dietary supplement products sold in New York, allegedly to expose potential cases of fraud.11-13 The study generated much discussion after finding that only 21% of products contained DNA of the claimed plant species, thereby concluding that the other 79% of the products were not in compliance with their labels. This report also raised the question as to whether DNA barcoding and DNA-based authentication methods are sufficient and/or conclusive for the determination of botanical identity, and to what level DNA methodology addresses Botanical Integrity.
What is the role of plant DNA authentication in Botanical Integrity?
DNA-based methods such as DNA barcoding are one of the many available tools that can be used in botanical examination (“botany,” See Fig. 1). The key outcome of botanical examination is the positive identification of the plant or plant-derived material by a scientifically valid method; the effectiveness of botanical assays based solely on DNA authentication depends on the presence of tissue and/or DNA from the target plant. In addition to DNA barcoding, there are other useful methods of botanical examination, such as morphology with the use of taxonomic keys, microscopy, and organoleptic analysis. In addition, a myriad of well-established methods are available to perform phytochemical analyses (e.g., high-performance TLC [HP-TLC], liquid chromatography [(ultra-HP or HP)LC-UV, LC-MS], NIR spectroscopy, MS, tandem MS [MS/MS], and NMR) and bioactivity assessments (e.g., in vitro and in vivo assays for a variety of biological activities such as estrogenicity,14 chemopreventive,15,16 and anti-inflammatory activities17). Importantly, chemical and biological assays can provide strong evidence about Botanical Integrity even when original plant tissue or DNA is absent, such as in botanical extracts or final products containing them, or when the test materials contain other constituents such as excipients that may confound the analytical challenge. All three types of methodologies together (botany, chemistry, bioactivity) establish Botanical Integrity, and it is incomplete unless adequate phytochemical analyses have been performed. The complex chemical composition of botanicals requires parallel bioanalytical approaches for adequate description, especially when considering that the bioactive constituents in botanicals are (phyto)chemicals.
The assurance of Botanical Integrity is of paramount importance to ensure safe and efficacious botanical-derived ingredients that can support both high-quality products and scientific excellence. Claims made about the quality, or lack thereof, of botanicals can be valid only if they rest on a variety of established and validated analytical methods (botany, chemistry, bioactivity) that are sufficient to address Botanical Integrity (See Fig. 1). In our experience, the employed methods have to be complementary to each other and cover at least two (botany and chemistry) — if not all three — of the major domains of expertise, leading to botanically, chemically, and biologically standardized materials.18 Notably, this process can be quite demanding, considering that several different (orthogonal) methods should be used to cover each domain of expertise.
In our scientific judgment, performing just one form of analysis (e.g., DNA barcoding) is insufficient to fully assess Botanical Integrity. Single-track approaches generally lack the rigor essential for authenticating the identity and adequately describing the biomedical integrity of the test material. DNA-based methods can be valuable tools for botanical evaluation in the early stages of the botanical supply chain. However, for the analysis of commercial botanical extracts and finished dietary supplement products, the numerous other methods belonging to the domains of phytochemical analysis and bioactivity assessment are much more meaningful and practical.
Acknowledgement
The authors acknowledge support by grant P50 AT000155 from the Office of Dietary Supplements and the NCCIH of the US National Institutes of Health. The contents are solely the responsibility of the authors and do not necessarily represent the views of the funding agencies.
About the UIC/NIH Center for Botanical Dietary Supplements Research
Established in 1999, the UIC/NIH Center for Botanical Dietary Supplements Research addresses issues of standardization, quality, safety, and efficacy of botanical dietary supplements used for women’s health. Founded by the late Norman Farnsworth, PhD, and directed by Dr. Richard van Breemen, the Center’s leaders, including Drs. Judy Bolton and Guido Pauli, take an integrated, collaborative approach to botanical research. Based on extensive preclinical studies involving botany, chemistry, and biology, the Center has completed clinical studies with black cohosh (Actaea racemosa, Ranunculaceae), red clover (Trifolium pratense, Fabaceae), and hops (Humulus lupulus, Cannabaceae), specifically addressing botanical safety and efficacy. Educating the next generation of pharmacognosists remains a primary goal of the Center, which has trained more than 60 pre- and post-doctoral researchers.
References
- NCCIH policy: natural product integrity. National Center for Complementary and Integrative Health website. Available at: https://nccih.nih.gov/research/policies/naturalproduct.htm?lang=en. Accessed April 2, 2015.
- Hebert PDN, Gregory TR. The promise of DNA barcoding for taxonomy. Systemat Biol. 2005;54(5):852-859.
- Ajmal Ali M, Gyulai G, Hidvégi N, et al. The changing epitome of species identification – DNA barcoding. Saudi J Biol Sci. 2014;21(3):204-231.
- Chen S, Pang X, Song J, et al. A renaissance in herbal medicine identification: From morphology to DNA. Biotechnology Advances. 2014;32(7):1237-1244.
- Li M, Cao H, But P-H, Shaw P-C. Identification of herbal medicinal materials using DNA barcodes. J Systemat Evol. 2011;49:271-283.
- Stoeckle M, Gamble C, Kirpekar R, Young G, Ahmed S, Little D. Commercial teas highlight plant DNA barcode identification successes and obstacles. Sci Rep. 2011;1:42.
- Baker D, Stevenson D, Little D. DNA barcode identification of black cohosh herbal dietary supplements. Journal of AOAC International. 2012;95(4):1023-1034.
- Little DP. Authentication of Ginkgo biloba herbal dietary supplements using DNA barcoding. Genome. 2014;57(9):513-516.
- Techen N, Parveen I, Pan Z, Khan I. DNA barcoding of medicinal plant material for identification. Current Opinion in Biotechnology. 2014;25:103-110.
- Kazi T, Hussain N, Bremner P, Slater A, Howard C. The application of a DNA-based identification technique to over-the-counter herbal medicines. Fitoterapia. 2013;87:27-30.
- Study: Many Herbal Supplements Aren’t What the Label Says. New York Times. February 3, 2015. Available at: www.nytimes.com/aponline/2015/02/03/us/ap-us-herbal-supplements-investigation.html?_r=0. Accessed April 2, 2015.
- Seres DS. The potential danger of dietary supplements. CNN. February 5, 2015. Available at: www.cnn.com/2015/02/05/opinion/seres-herbal-supplements/. Accessed February 15, 2015.
- ABC Says New York Attorney General Misused DNA Testing for Herbal Supplements, Should Also Have Used Other Test Methods as Controls [press release]. Austin, TX: American Botanical Council; February 3, 2015. Available at: http://cms.herbalgram.org/press/2015/ABCSaysNYAttyMisusedDNA.html. Accessed February 10, 2015.
- Hajirahimkhan A, Simmler C, Yuan Y, et al. Evaluation of estrogenic activity of licorice species in comparison with hops used in botanicals for menopausal symptoms. PLoS ONE. 2013;8(7):e67947.
- Dietz B, Liu D, Hagos GK, et al. Angelica sinensis and its alkylphthalides induce the detoxification enzyme NAD(P)H:quinone oxidoreductase 1 by alkylating KEAP1. Chem Res Toxicol. 2008;21:1939-1948.
- Dietz B, Hagos GK, Eskra JN, et al. Differential regulation of detoxification enzymes in hepatic and mammary tissue by hops (Humulus lupulus) in vitro and in vivo. Mol Nutr Food Res. 2013;57(6):1055-1066.
- Hajirahimkhan A, Dietz BM, Bolton JL. Botanical Modulation of Menopausal Symptoms: Mechanisms of Action? Planta Medica. 2013;79(7):538-553.
- Farnsworth NR, Krause EC, Bolton JL, Pauli GFF, van Breemen RB, Graham JG. The University of Illinois at Chicago/National Institutes of Health Center for Botanical Dietary Supplements Research for Women’s Health: from plant to clinical use. Am J Clin Nutr. 2008;87(2):504S-508S.
|