Issue:
112
Page: 40-45
Value Chains of Botanical and Herbal Medicinal Products: A European Perspective
by Anthony Booker, PhD, Michael Heinrich, Dr. rer. nat. habil.
HerbalGram.
2016; American Botanical Council
By Anthony Booker, PhD,1,2 and
Michael Heinrich, Dr. rer. nat. habil.1
1 Research Cluster Biodiversity and Medicines/Centre for
Pharmacognosy and Phytotherapy, University College London (UCL) School of
Pharmacy, University of London
2 Division of Herbal and East Asian Medicine, Department of
Life Sciences, University of Westminster, London
Introduction
In recent years, the quality of botanicals has come under
increased scrutiny. Despite the availability of numerous high-quality products
from reputable companies, health care professionals, patients, and consumers
are understandably concerned about questionable botanical ingredients in
various consumer products. In Europe, this includes products that are generally
unlicensed and unregistered supplements (also referred to as “botanicals”),
which are often poorly regulated, or even totally unregulated, depending on the
country of jurisdiction.*
This overview discusses the concept of value chains (some
have referred to them as “value networks”2), emphasizing that it offers a
framework for assessing the current quality problems with botanical raw
materials and extracts with respect to their adulteration, and for developing strategies
for best practices in the global botanical industry. We propose that some form
of self-regulation, if it enforces good quality and follows best practices,
will need to be linked to an understanding of value chains by those selling
final products, irrespective of their regulatory status. Importantly, the
concept of value chains also involves an understanding of the socioeconomic
impact of different production systems on primary producers and other
stakeholders.
The examples discussed here are based on studies of
plant-based products sold in Europe (and, to a lesser degree, in North America)
and compares regulated products with unregulated products, since such
distinctions apply in Europe, per below.
The Need for Value Chains
An important difference between, for example, the United
States and member states of the European Union (EU) is the level of regulation
of plant-based products and the subsequent enforcement of the regulations. The
European system offers an example of how quality assurance was introduced over
the last 15 years. In the EU, traditional herbal registration (THR), as
established by EU Directive 2004/24/EC,3 has set minimum standards that aspire
to guarantee the quality and safety of herbal medicinal products sold with
medical claims for minor self-limiting diseases. The THR is one of several
regulatory frameworks, and many European countries also regulate products under
the Well-Established Use Directive4 (Article 10(a) of EU Directive 2001/83/EC,
as amended) or under national regulations that allow for full licensing as
medicines.
THR is an example that highlights how consistent and
high-quality herbal medicines can be produced by using such a regulatory
framework. Alternatively, a company could go for a more rigorous and
well-enforced self-regulation, driven by the relevant industry bodies. The
introduction of more rigorous standards implemented at a global level results
in a greater integration between some processors and primary producers, who are
then able to ascertain better quality and to obtain more stable prices. Such
standards could be based on a regulatory framework or on commonly agreed-upon
principles of best practice, which are certified, for example, by an external
agency.
A useful framework to achieve this improved quality is the
concept of value chains, which for certain key food products (e.g., coffee [Coffea
arabica, Rubiaceae], tea [Camellia sinensis, Theaceae], and cocoa [Theobroma
cacao, Malvaceae]) have been investigated widely. However, value chains of
medicinal botanicals have been largely ignored in the global research
literature.5,6 Analysis of the value chains of botanicals is important, since
it is a critical part of understanding the quality and safety breakdowns that
can occur along the chain — breakdowns that are likely to result in
sub-standard or even unsafe finished products for consumers. While this is in
no way a new problem (in fact, pharmacognosy as a scientific discipline has
resulted, at least partially, from the need to identify adulteration and define
best quality), it has become an evermore relevant problem with the rapid and
dramatic globalization of trade in botanicals and herbal medicinal products.
A value chain differs conceptually from a supply or
commodity chain in that it is founded upon the insight that any company is more
than a random assembly of machinery, people, and finance. Only if these things
are arranged into definable systems will it become possible to produce a higher
quality commodity for which customers are willing to pay.7
In our own research on botanicals and regulated
phytomedicines, we have found that value addition can be introduced at various
stages of production. This can occur, for example, through certified organic
cultivation, use of superior extraction techniques, and/or through more
stringent regulation, such as the European THR.8-10
For example, in the case of saw palmetto (Serenoa repens,
Arecaceae) berry we found that regulated products manufactured using an
extraction process with a soft gelatin capsule dosage form were typically of
higher quality and more consistent than other products tested.11 In the case of
Rhodiola rosea (Crassulaceae) root products, we found that approximately one
quarter of products that were marketed without a THR were of poorer phytochemical
quality than those marketed with a THR as Traditional Herbal Medicines (THMs).10
In addition, some of these products were adulterated with other species or did
not meet their label specifications. This does not mean that all non-THR
products were of poor quality. In fact, some sold as botanicals were
comparable. One major problem with non-THR products is that it is practically
impossible for consumers to differentiate high-quality products from
low-quality products. Our data confirm that by choosing a THR product, the
quality and safety are assured.
Benefits of Vertical Integration
We suggest that vertical integration, in which contracts are
made directly with the farmers and primary processors, not only benefits those
involved in primary production but also can lead to higher quality products for
consumption in more economically developed countries. In such a value chain, a
lead organization is responsible for two or more intertwined steps of the
manufacturing or value chain process. Vertical integration, however, has been
criticized as resulting in a high degree of dependence on the primary
producers.9
The product quality and economic benefits for a primary
producer are highlighted in our work on turmeric (Curcuma longa,
Zingiberaceae).12 Looking at the production process of a multipurpose product
containing turmeric root and rhizome, we found that turmeric derived from
organically grown crops in India under contract from a European manufacturer
retained more volatile phytochemical compounds. Such compounds are generally
lost when turmeric is stored for long periods (e.g., when the market price is
low). Moreover, we found that some products that were obtained through
middlemen were the wrong species and thus did not meet the label claims (Figure
1).
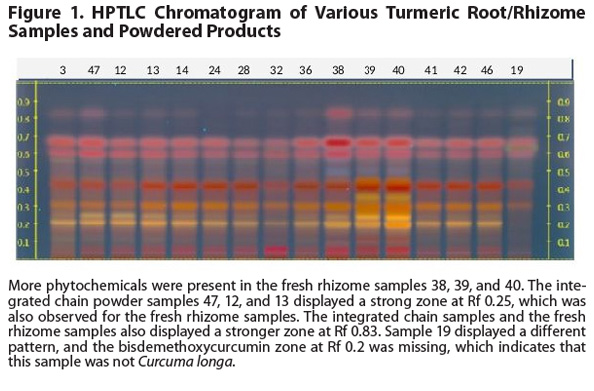 Products derived from a vertically integrated value chain
(VIVC) have obvious benefits for the consumer and, based on our assessment, the
producers as well. The producers we evaluated in India, who were able to access
the international market and agree to a quantity and price with the
manufacturer prior to cultivation, were less susceptible to the price
fluctuations and market shocks that can easily upset the smooth and expedient
transfer of goods from one country to another.12
Value chains of botanicals and herbal medicinal products can
be highly diverse. Vertical integration is not widespread throughout the
industry, and free markets and middlemen are still the most common routes of
supply. Supply chains for cultivated materials and wild-collected materials
each have associated challenges, and the picture is further complicated by a
lack of regulatory harmonization among different global regions and countries,
including many that are within the EU.
In our own investigations,10-12 we found that a VIVC was conducive
to the manufacture of organically grown products in which it is a requirement
that traceability to the exact area of origin can be proved, and documented
procedures for any primary processing are implemented (Figure 2).
THR vs. Non-THR Products
Multiple factors differentiate products produced with a THR.
Although there is no requirement for a THR product to be linked to any
particular value chain, a company’s commitment to and considerable financial
investment in obtaining a THR constitutes a significant value addition to the
product (Table 1), and it requires the establishment of robust systems of
quality control, from good agricultural and collection practices (GACPS) to
good manufacturing practices (GMPs) throughout the value chain.
THR products used to treat minor self-limiting conditions
are now widely available within the EU. It is a legal requirement for any
product that makes a medicinal claim, or any product that is deemed to have a
significant pharmacological effect, to hold a registration before being placed
on the market.13 Such products can be readily identified by their unique THR
number, and many also display the THR logo.
Food supplements (botanicals), however, are not yet required
to have a THR before entering the market in Europe, and they remain widely
unmonitored. Although regulations do exist, they are rarely cited or enforced.
Food supplements are often mislabeled or make misleading claims, and the lack
of effective enforcement in Europe has resulted in a large number of these products
entering the market.
The value chains of food supplements typically are
undisclosed, and it is only through careful analysis of the products that
problems may be detected. In our unpublished investigation of milk thistle (Silybum
marianum, Asteraceae) fruit extract and our analysis of ginkgo (Ginkgo biloba,
Ginkgoaceae) leaf extract products,14 carried out in collaboration with the BBC
for its series Trust Me, I’m a Doctor,15 we found widespread quality and
adulteration problems. Approximately 40% of unregistered milk thistle products,
sold legally as supplements in the EU, contained very low levels of the
bioactive marker compounds collectively referred to as silymarin. Furthermore,
after a detailed investigation, we found that more than 50% of ginkgo products
either were not compliant with their label claims; contained high levels of
flavonols, rutin, and/or quercetin; or were adulterated, one with a
5-hydroxytryptophan (5-HTP) derivative.
Wild-collected vs. Cultivated Material
The impact that different value chains have on botanicals
and herbal medicinal products and their quality also can be linked to
livelihoods and sustainability. Many rural communities and indigenous groups,
particularly in Asia, depend on medicinal plant collection for their livelihoods.16
A common pattern can be seen across different countries: As collected plants
are depleted in the wild, their scarcity in the marketplace increases along
with their economic value. This drives collectors to travel to isolated and
potentially dangerous areas in order to find more of the raw material, or to
use superficially similar material that can be sourced at lower cost. The
collectors themselves, however, are often unaware of the true market value of
the plants they collect and are prone to exploitation by middlemen.
The pressure to find more material may lead to adulteration
of the crop with similar species (e.g., as with Rhodiola species) or
adulteration further down the value chain. Another example is the adulteration
of ginkgo products with lower-cost rutin derived from buckwheat (Fagopyrum
esculentum, Polygonaceae). This is done in order to increase the flavonoid
content of the product and to achieve a superficial similarity to some of the
existing analytical standards that relate to ginkgo leaf extract. Clearly all
such products, if they do not comply with the label claim, are put on the
market illegally.
An integrated chain using cultivated material may provide a
better alternative to these models, but a major drawback for many manufacturers
and retailers is the cost. Cultivated material is typically more expensive than
wild-collected material, especially when it is produced in more economically
developed countries. This is particularly true for root crops that require a
number of years of growth before they can be harvested, such as Asian ginseng (Panax
ginseng, Araliaceae) and rhodiola. These crops are typically grown for four to
six years before harvesting and incur considerable expenditure of both time and
money. There are also concerns by some that cultivated material may be in some
way inferior to wild-collected material, and that certain cultivated
plant-based products may be less appealing to consumers.
Welfare Effects
Some companies, including manufacturers of food supplements
containing botanical ingredients, have managed not only to find a way to
cultivate their own crops, but also to use the VIVC model to their advantage.
In doing so, they highlight to the general public the high quality of their
products due to the tight controls employed along the chain, and raise
awareness of the potential welfare effects that such chains can have on the
primary producers in less economically developed countries (e.g., in India and
Eastern European countries).17 These welfare effects may not be sizeable in
terms of hard cash but can help in some practical ways, and will be of
longer-term benefit. For example, farmers can plan their crops based on
definite orders, which can give farm workers and those involved in primary
processing fixed terms of employment, rather than having to travel the country
in search of farm labor or factory-based employment.
The VIVC model has some obvious similarities to the
Fairtrade approach.* However, in order to gain Fairtrade certification, farmers
need to have a certain amount of infrastructure already in place. Consequently,
it is not always a suitable partnership for the poorest farmers.18 The
partnerships that have been built between farmers and companies producing both
regulated and unregulated end products may offer an extra level of support for
some of these poorest workers. Of course, a more informal fair-trade VIVC
approach can also have its dangers without good governance. Some studies
suggest that certain VIVCs, particularly ones that are dominated by a single
powerful company, can lead to negative effects on the livelihoods of small
producers.19 Only through the establishment of mutual trust over a period of
time (quasi-vertical integration) can these partnerships be successful and
flourish.
A comparison of the different value chain approaches along
with their main risks and benefits is given in Table 2. In comparing both
tables, it becomes apparent that VIVCs and THR-driven supply systems have a
number of similarities, most importantly as they relate to the possibility of
ascertaining a consistent, high-quality end product.
Conclusion
Overall, to both producers and consumers, VIVCs appear to
offer some distinct advantages over the reliance on middlemen that often occurs
in a free-market system. However, in a market in which cost may still be the
major driving force for most consumers of botanicals and herbal medicinal
products, it is unlikely that VIVCs will become commonplace without sufficient
consumer-driven demand for a specific group of products (e.g., demand for
organically grown raw materials).
The examples presented here offer models for how to
ascertain the best quality botanicals and herbal medicinal products. The
research highlights not only the need for taking a broader approach with regard
to quality control (including an understanding of best practices from source to
consumer), but also that either regulation or self-regulation of the relevant
industry is essential for quality assurance.
The lack of any government strategies for the effective cultivation,
process-management, and regulation of wild-sourced medicinal or locally grown
(often by smallholders) plant material — particularly plant material
originating from less economically developed countries — is a major cause for
concern for producers, manufacturers, and consumers of plant-based products.
However, VIVC-based approaches, which encompass effective partnerships, ethical
trading, and good governance, can help provide a more stable platform from
which safe and high-quality products can be sustainably produced.
Acknowledgements
The initial research carried out on value chains and
turmeric, which allowed the authors to develop the overarching concepts, was
funded by The Leverhulme Trust (UK). Anthony Booker’s research position is
funded through a charitable donation by Dr. Willmar Schwabe GmbH & Co.
KG, Karlsruhe, Germany.
Research on saw palmetto was partially funded by Bioforce
AG, Switzerland. Intact and unopened ginkgo and milk thistle product samples
were purchased with funds provided by the BBC. The authors express special
gratitude to Deborah Johnston, PhD (School of Oriental and African Studies,
University of London), for many discussions during the development of the
concepts on value chains.
Anthony Booker, PhD, is a senior lecturer in Chinese herbal
medicine and medicinal plant science at The University of Westminster in
London. In 2014, he received a doctorate from University College London in
ethnopharmacy and pharmacognosy. His current post-doctoral projects at UCL
investigate medicinal plant value chains and the quality of plant-based
products. Email: anthony.booker.11@ucl.ac.uk.
Michael Heinrich, Dr. rer. nat. habil., is a professor at
the Centre for Pharmacognosy and Phytotherapy, School of Pharmacy, University
of London. Much of his research interest lies in ethnobotany, pharmacognosy,
the quality of herbal medicinal products, and designing increased value for
herbs along the supply chain. He is the co-author of a textbook on pharmcognosy
and a member of the ABC Advisory Board. Email: m.heinrich@ucl.ac.uk.
*The American Botanical Council (ABC) has been actively
bringing together stakeholders interested in educational efforts intended to
help reduce the level of adulteration and contamination of ingredients used in
such products.1
*Fairtrade (www.fairtrade.net) was established to support farmers and farm workers, mainly in less economically developed countries. The Fairtrade mark gives consumers the knowledge that workers have been paid a fair price and work under a set of agreed-upon working conditions.
Sidebar: Key Findings from UCL Investigations of Botanicals
and Herbal Medicinal Products
In 2010, the research group of Michael Heinrich, Dr. rer.
nat. habil, at the UCL School of Pharmacy in London initiated a series of
investigations of the authenticity and identity of various herbal medicinal
products and herbal food supplements sold in the United Kingdom and links to
different value chains of such products.
In our initial project, we investigated value chains of
turmeric, which is grown as a cash crop throughout India.12 It is generally
sold at auction, and the price can be variable from one year to the next. When
the price is high one year, it encourages farmers to plant more, frequently
resulting in an excess of crop the following year, which usually leads to a
fall in the price. This is pure supply-demand economic dynamics. When the price
is low, farmers may store the dried crop rather than sell it. The dried
turmeric rhizomes can sometimes be stored for years in poor conditions, and
farmers can rely on heavy use of pesticides and other chemicals to keep the
material from degrading or perishing. Our analytical data suggested that this
long-term storage also can result in the loss of therapeutically important,
mainly volatile compounds (e.g., tumerone) that can evaporate over time.
We also investigated rhodiola products (i.e., herbal
products made from roots of plants in the genus Rhodiola). These included both
registered herbal products and unregistered food supplements used for the
prevention or treatment of fatigue and widely used to improve sports
performance. We looked at 39 products available in health food stores and on
the internet and found that approximately 25% of these products were of poor
quality.10 The main problem was that an incorrect species had been used.
Instead of the preferred R. rosea — the species of Rhodiola that has the most
clinical research — the lower-cost Chinese species R. crenulata had been
substituted in its place, even though 34 products claimed on the label to
contain only R. rosea. Although R. crenulata is used medicinally in China, it
does not contain the important compounds that give R. rosea its reputation as
an effective adaptogenic medicinal product. More troublesome, a selection of
the samples appeared to contain no Rhodiola species, and one was found to
contain 5-HTP, a naturally occurring compound with reputed antidepressant
properties. All of the THR products (n = 10) complied with their label
specifications.
With ginkgo and milk thistle, we used a similar sampling
strategy to rhodiola, using the internet, visiting high street supermarkets,
pharmacies, and health food shops, and obtaining a reasonably representative
sample of products available to UK consumers (ginkgo: n = 35; milk thistle: n =
18). Once again we found that 22% of all milk thistle products and 25% of all
ginkgo products were of very poor quality when compared to the reference
material or to THR products.14 The quality problems included low concentrations
of key compounds (with some being almost undetectable) when compared to
reference products commonly used in intervention and clinical studies, and
adulteration with other substances. Since the original work was done, we have
looked more closely at the ginkgo samples and suggest that more than 50%
contain low levels of ginkgo, and thus also are poor quality products. Some
ginkgo samples appeared to have greater quantities of rutin than were
detectable in the reference material. This suggests that they had been “spiked”
— a process in which exogenous rutin is added to increase the total flavonoid
content, making it appear that the samples are of acceptable quality. And again
we found that one product contained a 5-HTP-related compound. This product was
from the same company as the adulterated rhodiola product.
References
- ABC-AHP-NCNPR Botanical Adulterants Program. Available at: http://cms.herbalgram.org/BAP/index.html. Accessed September 29, 2016.
- Bewicke C. The global botanical supply chain: How it works & do we get what we pay for? — A supplier’s viewpoint. Talk presented at: The UNPA Raw Materials & Supply Chain Summit; February 23-24, 2016; Salt Lake City, UT. Available at: www.slideshare.net/SandyHays/unpa-2016-the-global-botanical-supply-chain-by-cal-bewicke-eni. Accessed September 29, 2016.
- European Parliament and the Council of the European Union. Directive 2004/24/EC of the European Parliament and of the Council of 31 March 2004 amending, as regards traditional herbal medicinal products, Directive 2001/83/EC on the Community code relating to medicinal products for human use. Official Journal of the European Union. April 30, 2004;L 136:85-90.
- European Parliament and the Council of the European Union. Directive 2001/83/EC of the European Parliament and of the Council of 6 November 2001 on the Community code relating to medicinal products for human us
- Fullbright. Product chain study: Orthodox tea. Commercial agriculture development project. Vol Final Report. Nepal: Ministry of Agriculture and Cooperatives, Department of Agriculture; 2008:1-31.
- Kaplinsky R. Competitions policy and the global coffee and cocoa value chain. United Nations Conference for Trade and Development (UNCTAD): United Nations; 2004.
- Recklies D. The Value Chain. Management Models and Tools. Vol 2013. Recklies Management Project GmbH; 2001. Available at: www.themanager.org/Models/ValueChain.htm. Accessed July 31, 2016.
- Booker A, Johnston D, Heinrich M. Value chains of herbal medicines — Research needs and key challenges in the context of ethnopharmacology. J Ethnopharmacol. 2012;140:624-633.
- Shahidullah AKM, Haque CE. Linking medicinal plant production with livelihood enhancement in Bangladesh: Implications of a vertically integrated value chain. J Transdisciplinary Environ Stud. 2010;9:1-18.
- Booker A, Jalil B, Frommenwiler D, et al. The authenticity and quality of Rhodiola rosea products. Phytomedicine. 2016;23:754-762.
- Booker A, Suter A, Krnjic A, et al. A phytochemical comparison of saw palmetto products using gas chromatography and H1 NMR metabolomic profiling. Journal of Pharmacy and Pharmacolgy. 2014;66:811-822. doi:10.1111/jphp.12198.
- Booker A, Johnston D, Heinrich M. The welfare effects of trade in phytomedicines: A multi-disciplinary analysis of turmeric production. World Development. 2016;77:221-230.
- EMA. Uptake of the traditional use registration scheme and implementation of the provisions of Directive 2004/24/EC in EU member states. Patient Health Protection. London, UK: European Medicines Agency; 2013.
- Booker A, Frommenwiler D, Reich E, Horsfield S, Heinrich M. Adulteration and poor quality of Ginkgo biloba supplements. J Herbal Med. 2016;6(2):79-87.
- Do herbal supplements contain what they say on the label? Trust Me, I’m a Doctor. BBC Two website. Available at: www.bbc.co.uk/programmes/articles/4hX30rMYkMv9YjMTH38MY6/do-herbal-supplements-contain-what-they-say-on-the-label. Accessed October 4, 2016.
- Alam G, Belt J. Developing a medicinal plant value chain: Lessons from an initiative to cultivate Kutki (Picrorhiza kurrooa) in Northern India. KIT Working Papers Series C5. Amsterdam: KIT; 2009.
- Netherlands: Springer; 2006:191-237.
- van de Kop P, Alam G, de Steenhuijsen Piters B. Developing a sustainable medicinal plant chain in India. Linking people, markets and values. In: Ruben R, Slingerland M, Nijhoff H, eds. Agro Food Chains and Networks for Development. Vol
- Nelson V, Pound B. The Last Ten Years: A Comprehensive Review of the Literature on the Impact of Fairtrade. Natural Resources Institute (NRI); University of Greenwich: 2009.
- van Niekerk J, Wynberg R. The trade in Pelargonium sidoides: Rural livelihood relief or bounty for the ‘bio-buccaneers’? Devel So Africa. 2012;29(4):530-547.
|