Issue:
112
Page: 62-69
Adulteration of Pomegranate Products — A Review of the Evidence
by John H. Cardellina II, PhD, Mark Blumenthal
HerbalGram.
2016; American Botanical Council
Editor’s note: This article was produced as part of the
ABC-AHP-NCNPR Botanical Adulterants Program’s continuing coverage of
adulteration-related issues.
Introduction
Pomegranate (Punica granatum, Lythraceae) fruit juice has
enjoyed considerable market growth and commercial success as a popular beverage
in the United States and internationally for more than a decade. The
consumption of pomegranate juice in the United States went from roughly 75
million eight-ounce servings in 2004 to about 450 million servings in 2008 — a
500% increase.1 One review indicates that sales of pomegranate juice grew
dramatically from $84,500 in 2001 to $66 million in 2005.2 According to 2014
estimates, 150,000-200,000 metric tons of fresh pomegranates and 3.7 million
gallons of pomegranate juice concentrate are sold annually (A.R. Rejaei,
director of clinical regulatory affairs at POM Wonderful, email to M.
Blumenthal, April 7, 2015).
As the popularity of pomegranate has increased, many
suppliers of herbs and other plant-based materials have begun to produce a
variety of dried pomegranate materials (e.g., dried juice concentrates and
extracts) for use as ingredients with health-promoting properties in the
burgeoning global market for natural products.* These concentrates and extracts
are produced by various means from pomegranate juice, whole pomegranate fruit,
or selected parts of the fruit.
Many manufacturers produce botanical extracts standardized
to a chemical compound or a class of compounds (marker compounds) for quality
control purposes and/or to help ensure consistent, reproducible biological
activity. Following this trend, some manufacturers of pomegranate fruit extracts
(PFEs) are standardizing their PFEs to ellagic acid (EA), a common phenolic
compound widely distributed in nature. EA has a number of reported beneficial
physiological activities, with much work focusing on the compound’s antioxidant
activity.3 (EA was recently found to have pro-oxidant properties as well.) The
antioxidant activities of EA metabolites formed in the intestinal tract (“colonic
metabolites,” such as urolithin A) are thought to be responsible for the
therapeutic effects attributed to EA.4,5
A number of PFEs are marketed with claims of high levels of
EA (ca. 40-70%), but industry experts have questioned the practice of
standardizing PFEs to such high levels of EA. A concern raised by some experts —
and a central question in this paper — is whether such high EA levels are
endogenous in the pomegranate fruit source of these extracts, or if they are
the result of added, non-pomegranate-sourced (i.e., exogenous) EA.
Other pomegranate products have been the subject of
adulteration reports as well. A 2009 article describing pomegranate juice
adulterated with other fruit juices suggests that there may be several forms of
adulteration that exist in the marketplace.6
This article briefly reviews the chemistry and reported
health benefits of pomegranate, the evidence for adulteration of pomegranate
juice and extract, the pharmacology and safety of EA, and the issues
surrounding standardizing PFEs to EA content.
Background
Pomegranate is believed to have originated in an area
encompassing what is now Iran, Afghanistan, and northern India. Pomegranate
played a prominent role in Greek mythology, symbolism, and ceremonies, as well
as in numerous religions, including Zoroastrianism, Buddhism, early
Christianity, Hinduism, and Islam.7,8 All parts of the pomegranate plant (root,
bark, leaves, flowers, and fruit) have been used in Ayurvedic medicine in India
for a variety of purposes, including as an antiparasitic agent and blood tonic,
and for the treatment of ulcers, canker sores, and diabetes.7 The antitumor,
antidiabetic, cardioprotective, antioxidant, and antimicrobial properties of
pomegranate preparations have taken on a more prominent role in current use.2,9-12
The chemistry of pomegranate is dominated by phenolic
compounds of differing complexity, including benzoic and cinnamic acid
derivatives, flavonoids, ellagitannins, and anthocyanins.10 Lignans were
reported in pomegranate for the first time in 2009, adding to the family of
phenolics found in this species.13 Two analyses of pomegranate juice using
ultra high-performance liquid chromatography-mass spectrometry (UHPLC-MS) have
cataloged 67 and 75 different secondary metabolites, respectively, most of them
phenolics.14,15 Triterpenes have also been reported in pomegranate.16
Pomegranate seed oil contains phytosterols and has a fatty acid profile
dominated by punicic acid, an omega-5 linolenic acid isomer with the
carbon-carbon double bonds at positions 9, 11, and 13.17
The majority of pomegranate dietary supplements are
reportedly manufactured from dried extracts. The materials of commerce
discussed in this review are all derived from the fruit, whether from the seed,
juice, or rind/husk/peel.18 Pomegranate dietary supplements are typically
produced from either dehydrated pomegranate juice (also known as juice
concentrate, not an extract) or compounds extracted from pomegranate fruit
and/or fruit parts by using a solvent (e.g., water, ethanol, methanol, or
combinations thereof), often after the juice has been removed by mechanical
pressing. Depending on the methods used and the desired chemical profile of the
finished material, manufacturers often try to preserve the bioactive phenolic
compounds19 of pomegranate that have been the subject of numerous chemical,
pharmacological, and clinical studies.
As a conventional food, pomegranate juice remains a popular
beverage for its antioxidant activity. A recent review summarizing the impact
of processing on the bioactive constituents, flavor, and aroma of pomegranate
juice noted that temperature, pH, pressure, and time all affect the final
concentration and ratios of different juice constituents.20
Evidence of Adulteration
At least three different forms of adulteration have been
reported in pomegranate products in the global marketplace: pomegranate juices
made with juice(s) from other fruits; pomegranate extracts spiked with
additional EA or polyphenols; and products made mostly from unknown or
unidentified source materials, with little to no pomegranate constituents.
Juice
Increased demand for an agricultural commodity in relatively
fixed supply usually drives up the cost of the raw material, which can tempt
less scrupulous suppliers and manufacturers to dilute or substitute the actual
commodity with lower-cost, more readily available materials. The addition of
lower-cost, readily available juices to more expensive juices in limited supply
has been an issue in the food and beverage industry for some time.21 Both
pomegranate juice and extracts thereof appear to have been subject to this form
of economically motivated adulteration.
In 2009, Zhang et al.6 evaluated 45 juice samples from 23 US
manufacturers, all purchased in local markets. First, they adapted criteria for
determining the identity of genuine pomegranate juice from the databases of
Krueger Food Laboratories, Inc., and the Association of the Industry of Juices
and Nectars of the European Economic Community.22-24 After examining the
profiles of anthocyanins, ellagitannins, sugars, and acids in those juice
samples, they found that only approximately 35% of the tested juices had
complete profiles appropriate for or representative of pomegranate juice. (The
researchers also tested the samples for mannitol and tartaric acid content, and
used stable isotope ratio analysis [SIRA] to test for any added sugars.) This
seminal study established that adulteration of pomegranate juice was pervasive
at the time, and offered a multi-parameter profile to determine if juice
products are adulterated.
Numerous methods and criteria have been used previously to
identify adulterated juice products. In 2011, concern about fruit juice
adulteration led the Grocery Manufacturers Association to conduct an HPLC-MS
analysis of various fruit juices, including pomegranate, apple (Malus spp.,
Rosaceae), orange (Citrus sinensis, Rutaceae), red grape (Vitis vinifera,
Vitaceae), white grape (V. vinifera), and cranberry (Vaccinium macrocarpon,
Ericaceae), to determine their levels of tartaric, quinic, malic, and citric
acids.25 The researchers used the unique ratio of acids in each juice to
determine if other juice(s) were present. This study also confirmed the
presence of low levels of tartaric and quinic acids in pomegranate. A group in
Spain subsequently evaluated pomegranate juice to measure the contents of
organic acids, sugars, minerals, proline, and volatile flavor/aroma compounds.
The analyses revealed adulteration with varying amounts of grape or peach (Prunus
persica, Rosaceae) juice.26 Furthermore, Tezcan et al. in Turkey used a chiral
micellar electrokinetic chromatography laser-induced fluorescence (MEKC-LIF)
method to develop a fairly complete amino acid profile of pomegranate juice.
The results suggested that asparagine (L-Asn) could serve as an effective
indicator of adulteration with apple juice, since it is six- to 13-fold more
abundant in juices made from certain varieties of apples.27
Borges et al. used HPLC with multiple detectors in two
studies of products advertised to contain “100% pomegranate juice.” In one
study,28 HPLC coupled with a photodiode array and tandem mass spectrometers
(HPLC-PDA-MS2) was used to analyze the polyphenolic profiles of 36 commercially
sold juices, including six labeled to contain 100% pomegranate juice and 20
pomegranate juices blended with other fruit juices. (The remaining 10 juices
were composed of other fruits.) Three of the “pure” pomegranate juices
exhibited typical pomegranate profiles and the highest ellagitannin contents of
the tested juices, but only one of these contained significant concentrations
of anthocyanins. The other three juices advertised as “pure pomegranate juice” displayed
aberrant HPLC profiles relative to the expected pomegranate fingerprint, which
suggested blending with other fruit juices or the addition of exogenous
polyphenols.
In the second study by Borges et al., fluorescence detection
(FD) was combined with the previously used HPLC-PDA-MS method to compare the
polyphenolic profiles of a known pure pomegranate juice to red wine and three
other juices claiming to be pure pomegranate juice.29 The red wine and the
authentic pomegranate juice both exhibited the expected anthocyanin profiles
and were readily distinguished by the analytical methods used (pomegranate
juice also produced peaks for its ellagitannins, while the red wine, in
contrast, gave peaks for flavan-3-ol monomers and procyanidin dimers and trimers).
Notably, the three supposedly pure pomegranate juices had the expected
ellagitannins, but they also exhibited anthocyanin profiles indicative of a
mixture of both source fruits (pomegranates and grapes), as well as the
procyanidins and flavan-3-ols seen in grape-derived juices. These results
suggested that grape juice might be used to dilute more expensive pomegranate
juice, while maintaining an appropriate color.
Krueger Food Laboratories followed up its aforementioned
work22,23 with a three-year study of more than 500 juice samples.30 With this
large dataset, researchers were able to use an iterative statistical analysis
to reduce the sample set to a group of compositionally consistent juices. This
was accomplished by calculating a mean and standard deviation of various juice
components for the whole set of samples, then excluding any samples that were
three or more standard deviations from the mean. The process was repeated on
the remaining samples until a stable set of 263 juices was obtained — the presumptive
authentic pomegranate juice samples. The report includes a table of 14
pomegranate juice components, mostly sugars and organic acids (with mean
content and standard deviation), and a representative HPLC-UV profile of the
anthocyanins. A number of patterns of adulteration were observed, including the
addition of up to seven fruit juices, sugars, anthocyanin colorants from many
natural sources, and artificial colors. Ten references are provided for the
analytical methods used, six of which are official AOAC International methods.
In 2016, researchers from Italy demonstrated the use of a
DNA-based method, Sequence Characterized Amplified Regions (SCAR), to detect as
low as 1% adulteration of pomegranate juice by 10 other botanical sources of
anthocyanins reported as potential adulterants of pomegranate products. The
SCAR marker selected as a positive control for pomegranate, designated ScPg231,
correctly identified eleven different accessions** of P. granatum. The marker
also identified pomegranate in four product mixtures: two herbal teas
containing 2% and 20% pomegranate, respectively; a jam containing pomegranate,
lemon (Citrus limon, Rutaceae), agave (Agave spp., Agavaceae), and pectin; and
a juice mix containing 3.5% pomegranate juice concentrate. These results
indicate that relatively short-length SCAR markers may be highly useful for
identifying pomegranate components with partially degraded DNA.32
Extracts
While pomegranate juice adulteration is generally the result
of addition of other fruit juices, adulteration of pomegranate extracts
predominantly involves the addition of exogenous polyphenolic material.
Pomegranate extracts not standardized to a particular marker, but instead to a
non-specific estimation of antioxidant capacity or total phenolics, may be
particularly prone to the addition of inexpensive polyphenols (e.g., EA) or
tannins to increase antioxidant activity, or to the addition of anthocyanins to
adjust color. In an extreme case, a non-pomegranate-based material could be
similarly augmented with polyphenols to resemble a pomegranate-derived product.
An analysis of the ellagitannin content and antioxidant
capacity of 27 commercially available pomegranate extracts found that only five
extracts contained significant amounts of punicalin and punicalagins
(pomegranate-specific ellagitannins). Seventeen of the samples contained mostly
EA, currently the most used marker compound for standardization of these
extracts, while the remaining five had little or no EA or ellagitannins and
weak antioxidant activity.33
Another analysis of 19 commercially available pomegranate
extracts by a different research group provided similar results.34 Qualitative
analysis indicated that only seven of the 19 tested extracts produced
polyphenolic profiles indicative of pomegranate, while 13 of the extracts had
EA levels exceeding what should be found in the arils (seed coverings) and rind
of pomegranate, and six of those had little or no pomegranate ellagitannins
present. The extracts that contained some pomegranate ellagitannins but no
pomegranate anthocyanins, the authors noted, were likely produced by extraction
of the press cake (rinds and arils) after juice production. Two possible
explanations were presented for the extracts with high levels of EA but no
pomegranate ellagitannins: (1) The extraction and/or associated processing
methods were so harsh that the ellagitannins were all decomposed, or (2) no
pomegranate was present, and exogenous EA was added.
In addition to the HPLC analyses described above, thin-layer
chromatography (TLC) may also be helpful in determining whether an unknown
sample matches authentic pomegranate reference samples.
Ellagic Acid
EA Standardization
The issue of standardizing PFEs to EA content is complex,
sometimes even problematic, for a number of reasons, discussed below.
1. Free EA is not the most abundant phenolic compound in
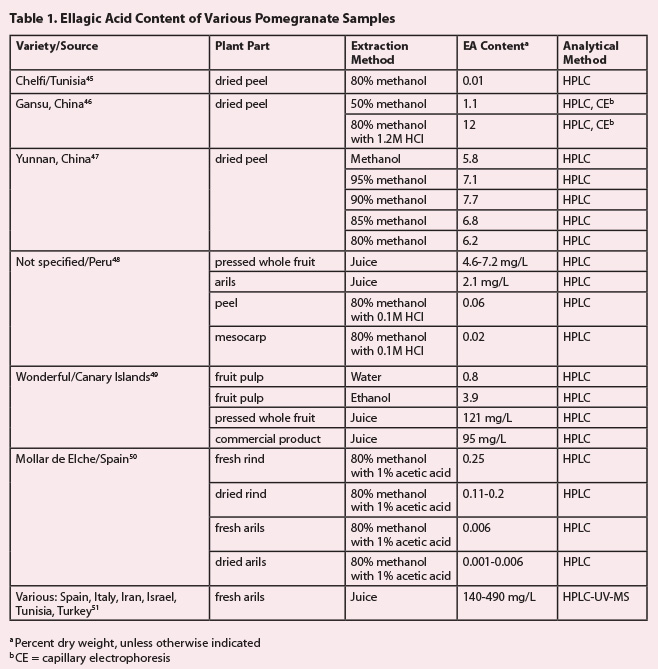
pomegranate.19 Table 1 summarizes the EA content of various pomegranate parts
from several geographic regions, illustrating that EA yields from experimental
pomegranate extracts are not likely to exceed 10%. Furthermore, the data
suggest that considerable variation of EA (and other polyphenols) may be
observed in different varieties of pomegranate, as well as in accessions of the
fruit from different ecological niches and geographic regions. Further
complicating the matter is the fact that different processing methods for the
juice or extracts can result in partial hydrolysis of EA esters
(ellagitannins), leading to higher observed levels of free EA in the resulting
extract. Considering the experimental conditions used in various publications
cited in Table 1, it would be exceedingly difficult, if not practically
impossible, to achieve the high levels of EA (40-70%) advertised in products
claiming to contain only pomegranate fruit.
One report has described the preparation of a processed
extract of pomegranate rinds containing a remarkable 90% EA, as measured by
HPLC analysis.35 The process was described as a triple extraction with hot 50%
ethanol, followed by boiling those extracts in hydrochloric acid (HCl) for six
hours, and drying the resulting reaction mixture solids. An extract processed
in this manner could be used to augment the EA content of pomegranate extracts
and products derived therefrom. However, extracts produced using these methods
would be unlikely to retain the chemical fingerprint of pomegranate
polyphenols.
2. EA is also available in substantial quantities from other
sources of ellagitannins (e.g., chestnut [Castanea spp., Fagaceae] bark or
fruit36 or gall nuts), offering a potentially lower-cost alternative source for
“enhancing” pomegranate extracts. Moreover, the last dozen years have seen a
surge of interest in producing free EA through the biotransformation of tannins
(i.e., the enzymatic degradation of complex polyphenols to EA by microbial
cultures).37-41
3. EA is not the primary or foremost bioactive compound in
pomegranate. In fact, the compounds responsible for many of the pharmacological
benefits attributed to pomegranate have not been adequately identified. It is
possible, even likely, that different compounds may be associated with various
bioactivities.
Punicalin and punicalagins A and B are more abundant than EA
in pomegranate and have been reported to account for 89% of the antioxidant
activity of pomegranate juice.19,42 Although standardizing PFEs to EA content
may be convenient from an analytical chemistry standpoint, it would be more
logical to standardize PFEs to punicalin and punicalagins A and B, which are
more abundant and distinctive markers for pomegranate. The availability of
high-quality reference standards to analyze for these potential markers might
be a short-term issue, but there is clearly a market need for such reference
standards.
4. EA is poorly soluble in aqueous media, particularly under
acidic conditions, and it is not significantly bioavailable when consumed as
the free acid.43,44 However, hydrolysable ellagitannins (e.g., punicalin and
the punicalagins) not only are more soluble than EA in physiological media, but
also yield bioavailable EA during metabolism in the human gut.44 Ironically,
higher EA content in various commercial products would thus seem likely to
result in lower blood levels of EA, relative to the complex of authentic,
unaltered pomegranate polyphenolic compounds.
EA Bioavailability
A single human volunteer, after fasting overnight, took a
dose of 180 mL of pomegranate juice containing 25 mg of EA and 318 mg
ellagitannins. Blood samples were drawn prior to dosing and at 30 minutes, one,
two, three, four, and six hours after dosing, and they were processed and
analyzed by HPLC. The peak EA concentration, 31.9 ng/mL (0.106 µM), was
observed in the one-hour sample, and EA was undetectable by the fourth hour.
The ellagitannins were not detected at any time point.52
In another small pharmacokinetic study, 11 human volunteers
consumed 45 g of freeze-dried black raspberries (Rubus spp., Rosaceae) each day
for seven days. Blood and urine samples were collected and analyzed for four
anthocyanins and free EA.53 The research team found that less than 1% of the
compounds of interest were absorbed and eliminated in urine. Maximum levels of
the anthocyanins and EA occurred between one and two hours after dosing, while
the highest levels in urine were found up to four hours after consumption. No
adverse effects were noted.
In a different study, 11 volunteers fasted overnight before
consuming two capsules, each containing 400 mg of pomegranate extract. The
combined dose contained 21.6 mg of EA and 330.8 mg of ellagitannins. Blood was
drawn at 30 minutes, one, two, four, six, eight, and 24 hours after dosing, and
subsequently analyzed by HPLC-MS. Significant variation in blood levels was
observed among the 11 subjects, but the average peak EA concentration, 33.8
ng/mL, was recorded at the one-hour time point.54 These results compare
remarkably well with the one-person study reviewed above,52 even though the
pomegranate products used were in different dosage forms (juice vs. capsule)
and were produced by different manufacturers.
An additional study involved 16 adults who consumed,
sequentially, eight ounces of pomegranate juice from a first-press squeezing of
whole fruit, a teaspoon of a concentrated liquid extract of the fruit material
left after first press squeezing in eight ounces of water, and a capsule containing
1,000 mg of a dried powder obtained by a solid phase extraction of the
concentrated liquid extract described above. Consumption of each of these doses
was separated by a one-week washout phase. Blood samples were drawn prior to
dosing and at 30 minutes, one, two, three, four, six, and 24 hours after
dosing. The three test materials had similar gallic acid equivalent values, but
no analyses for punicalagins or EA were provided in the paper. The times to
maximum concentration (Tmax) of EA for the juice and extract dissolved in water
were similar to previously reported times, roughly one hour. The Tmax for the
encapsulated dry powder was considerably longer, about 2.6 hours, but this may
have been related to dissolution time of the gelatin capsule. All three dosage
forms gave similar areas under the curve (i.e., integration of the plot of EA
concentration vs. time after dosing).55
EA Safety
A search of the scientific and toxicological literature
revealed no reports of adverse effects or significant toxicity associated with
EA. The poor bioavailability of EA likely makes establishing a lowest-observed
adverse effect level (LOAEL) for EA difficult.
In an effort to determine an LD50 level (a measure of acute
toxicity that is based on the lethal dose that produces mortality in 50% of
test animals) in mice, the animals were given a single dose of 25-1,500 mg/kg
of EA orally or 25-1,000 mg/kg intraperitoneally and observed for 14 days. No
animals died within 24 hours, meaning that an LD50 was not reached. No animals
died after 14 days, nor were any irreversible signs or symptoms observed in the
test animals.56
In a subchronic toxicity study, rats were given an EA oral
dose of 10, 30, or 100 mg/kg. Thirty days after dosing, hematological and
biochemical tests were conducted on blood samples and histopathological
profiles were observed for vital organs. The only deviation from normal
observed was reduced uric acid levels at the 30 and 100 mg/kg doses. Thus, this
study found that free EA exhibited quite low toxicity.56
In another, longer subchronic toxicity study, rats were fed
a powder basal diet for 90 days that included EA at dose levels of 0, 1.25,
2.5, and 5% (0, 9.4, 19.1, 39.1 g/kg body weight, respectively, in males, and
0, 10.1, 20.1, 42.3 g/kg body weight, respectively, in females).57 No
mortality, histopathological changes, or treatment-related clinical signs were
observed, except for decreased weight gain in female rats fed the three actual
doses of EA. From these results, the authors calculated an estimated
no-observed adverse effect level (NOAEL) dose for EA in females of 3,254
mg/kg/day and estimated no-observed effect level (NOEL) values in males (3,011
mg/kg/day) and females (778 mg/kg/day).
This relatively small but consistent pool of data, along
with the widespread presence of EA in the human diet and the low
bioavailability of EA, suggest that high levels of EA in a supplement may not
be a safety issue, but rather a possible regulatory, efficacy, and/or ethical
matter.
Conclusion
The evidence reviewed above indicates that some pomegranate
juice products are adulterated by blending with other fruit juices and/or
colorants. Furthermore, some pomegranate juice concentrates and extracts (and
resulting products) are likely adulterated by the addition of non-pomegranate
EA sources. In either case, the consumer may not receive the expected benefits
of consuming pure, high-quality pomegranate juice or extracts.
Suppliers, buyers, and manufacturers of finished products
need to be aware of this issue and should take steps to ensure that their
materials are not adulterated. This report indicates that a single analytical
method will most likely not be able to determine all of the possible
adulterants (and approaches to adulteration) of pomegranate juice, juice
concentrate, and extract, but there is an array of available methods that can
be applied for quality control purposes. Ongoing research in various
laboratories will likely provide additional tools and methods in the near
future.
*Natural products may be referred to as functional foods,
dietary supplements (in the United States), natural health products (in
Canada), therapeutic goods (in Australia), or food supplements (in Europe),
depending on where they are sold.
**According to the US Department of Agriculture, an
accession is “a genetically unique plant sample from a particular geographic
location.”31
References
- Packer R.
Pomegranate juice adulteration. Food Safety Magazine. February 2013.
- Johanningsmeier
SD, Harris GK. Pomegranate as a functional food and nutraceutical source. Annu
Rev Food Sci Technol. 2011;2:181-201.
- Tomás-Barberán
FA, García-Conesa MT, Larrosa M, et al. Bioavailability, metabolism, and
bioactivity of food ellagic acid and related polyphenols. In: Recent Advances
in Polyphenol Research. Vol 1. Daayf F, Lattanzio V, eds. Hoboken, NJ:
Blackwell Publishing Ltd.; 2008:263-267.
- Espin JC,
Larrosa M, Garcia-Conesa MT, Tomas-Barberan F. Biological significance of
urolithins, the gut microbial ellagic acid-derived metabolites: the evidence so
far. Evid Based Complement Alternat Med. 2013(2013):270418.
doi:10.1155/2013/270418.
- Saha P,
Yeoh BS, Singh R, et al. Gut microbiota conversion of dietary ellagic acid into
bioactive phytoceutical urolithin A inhibits heme peroxidases. PLoS One.
2016;11:e0156811. doi:10.1371/journal.pone.0156811.
- Zhang Y,
Krueger D, Durst R, et al. International multidimensional authenticity
specification (IMAS) algorithm for detection of commercial pomegranate juice
adulteration. J Agric Food Chem. 2009; 57:2550-2557.
- Bhandari
PR. Pomegranate (Punica granatum L). Ancient seeds for modern cure? Review of
potential therapeutic applications. Int J Nutr Pharmacol Neurol Dis.
2012;2:171-184.
- Ruis AR.
Pomegranate and the mediation of balance in early medicine. Gastronomica.
2015;15:22-33.
- Miguel MG,
Neves MA, Antunus MD. Pomegranate (Punica granatum L.): A medicinal plant with
myriad biological properties — A short review. J Med Plants Res. 2010;4:2836-2847.
- Jasuja ND, Saxena R, Chandra S, Sharma R. Pharmacological
characterization and beneficial uses of Punica granatum. Asian J Plant Sci.
2012;6:251-267.
- Katz SR, Newman RA, Lansky EP. Punica granatum: Heuristic
treatment for diabetes mellitus. J Med Food. 2007;10:213-217.
- Jurenka J. Therapeutic applications of pomegranate (Punica
granatum L.): A review. Alt Med Rev. 2008;13:128-144.
- Bonzanini F, Bruni R, Palla G, Serlataite N, Caligiani A.
Identification and distribution of lignans in Punica granatum L. fruit
endocarp, pulp, seeds, wood knots and commercial juices by GC-MS. Food Chem.
2009;117:745-749.
- Calani L, Beghe D, Mena P, et al. Ultra-HPLC−MSn
(poly)phenolic profiling and chemometric analysis of juices from ancient Punica
granatum L. cultivars: A nontargeted approach. J Agric Food Chem.
2013;61:5600-5609.
- Mena P, Calani L, Dall’Asta C, et al. Rapid and
comprehensive evaluation of (poly)phenolic compounds in pomegranate (Punica
granatum L.) juice by uHPLC-MSn. Molecules. 2012;17:14821-14840.
- Jiang HZ, Ma QY, Fan HJ, et al. Fatty acid synthase
inhibitors isolated from Punica granatum L. J Brazilian Chem Soc.
2012;23:889-893.
- Kaufman M, Wiesman Z. Pomegranate oil analysis with emphasis
on MALDI-TOF/MS triacylglycerol fingerprinting. J Agric Food Chem.
2007;55:10405-10413.
- Gardner Z, McGuffin M, eds. American Herbal Products
Association’s Botanical Safety Handbook. 2nd ed. Boca Raton, FL: CRC Press;
2013:155-159.
- Gil MI, Tomás-Barberán FA, Hess-Pierce B, Holcroft DM, Kader
AA. Antioxidant activity of pomegranate juice and its relationship with
phenolic composition and processing. J Agric Food Chem. 2000;48:4581-4589.
- Nuncio-Jáuregui N, Calín-Sánchez A, Vázquez-Araújo L, Pérez-López
AJ, Frutos-Fernández MJ, Carbonell-Barrachin AA. Processing pomegranates for
juice and impact on bioactive components. In: Processing and Impact on Active
Components in Food. Preedy VR, ed. Amsterdam, Netherlands: Elsevier Inc.;
2015:629-636.
- US Food and Drug Administration Guide to Inspection of
Foods, Section 10, Orange and Other Juices. Available at:
www.fda.gov/ora/Inspect_ref/igs/foodspa.html. Accessed September 22, 2016.
- Krueger DA. Composition of commercial pomegranate juice.
Abstracts, 122nd Annual Meeting of the AOAC, 2008.
- Krueger DA. Composition of pomegranate juice. J AOAC Int.
2012;95:163-168.
- Association of the Industry of Juices and Nectars from
Fruits and Vegetables of the European Economic Community (AIJN). Draft
Reference Guideline for Pomegranate Juice, Code of Practice for Evaluation of
Fruit and Vegetable Juices. March 18, 2008.
- Ehling S, Cole S. Analysis of organic acids in fruit juices
by liquid chromatography-mass spectrometry: An enhanced tool for authenticity
testing. J Agric Food Chem. 2011;59:2229-2234.
- Nuncio-Jauregui N, Calin-Sanchez A, Hernandez F,
Carbonell-Barrachina AA. Pomegranate juice adulteration by addition of grape or
peach juices. J Sci Food Agr. 2014;94:646-655.
- Tezcan F, Uzaşçi S, Uyar G, Oztekin N, Erim FB.
Determination of amino acids in pomegranate juices and fingerprint for
adulteration with apple juices. Food Chem. 2013;141:187-1191.
- Borges G, Mullen W, Crozier A. Comparison of the
polyphenolic composition and antioxidant activity of European commercial fruit
juices. Food Funct. 2010;1:73-83.
- Borges G, Crozier A. HPLC-PDA-MS fingerprinting to assess
the authenticity of pomegranate beverages. Food Chem. 2012;135:1863-1867.
- Krueger Food Laboratories. Composition of pomegranate juice.
J AOAC International. 2012;95(1):163-168. Available at: www.kfl.com/pom.html.
Accessed June 2, 2016.
- Definitions: What is an accession? United States Department
of Agriculture – Agricultural Research Service website. Available at:
www.ars.usda.gov/plains-area/fort-collins-co/center-for-agricultural-resources-research/plant-germplasm-preservation-research/docs/ncgrp-faq/.
Accessed October 10, 2016.
- Marieschi M, Torelli A, Beghé D, Bruni R. Authentication of Punica
granatum L.: Development of SCAR markers for the detection of 10 fruits
potentially used in economically motivated adulteration. Food Chem.
2016;202:438-444.
- Zhang Y, Wang D, Lee RP, Henning SM, Heber D. Absence of
pomegranate ellagitannins in the majority of commercial pomegranate extracts:
implications for standardization and quality control. J Agric Food Chem.
2009;57:7395-400.
- Madrigal-Carballo S, Rodriguez G, Krueger CG, Dreher M, Reed
JD. Pomegranate (Punica granatum) supplements: Authenticity, antioxidant and
polyphenol composition. J Funct Food. 2009;324-329.
- Yoshimura M, Watanabe Y, Kasai K, Yamakoshi J, Koga T.
Inhibitory effect of an ellagic acid-rich pomegranate extract on tyrosinase
activity and ultraviolet-induced pigmentation. Biosci Biotechnol Biochem.
2005;69:2368-2373.
- Vekiari SA, Gordon MH, Garcia-Macias P, Labrinea H.
Extraction and determination of ellagic acid content in chestnut bark and
fruit. Food Chem. 2008;110:1007-1011.
- Vattem Dam Shetty K. Ellagic acid production and phenolic
antioxidant activity in cranberry pomace (Vaccinium macrocarpon) mediated by Lentinus
edodes using a solid-state system. Proc Biochem. 2003;39:367-379.
- Shi B, He Q, Yao K, Huang W, Li Q. Production of ellagic
acid from degradation of valonea tannins by Aspergillus niger and Candida
utilis. J Chem Technol Biotechnol. 2005;80:1154-1159.
- Juang W, Ni J, Borthwick AGL. Biosynthesis of valonia tannin
hydrolase and hydrolysis of valonia tannin to ellagic acid by Aspergillus SHL
6. Process Biochem. 2005;40:1245-1249.
- Robledo A, Aguilera-Carbó A, Rodriguez R, Martinez JL, Garza
Y, Aguilar CN. Ellagic acid production by Aspergillus niger in solid state
fermentation of pomegranate residues. J Ind Microbiol Biotechnol.
2008;35:507-513.
- Sepúlveda L, Ascacio A, Rodríguez-Herrera R, Aguilera-Carbó A,
Aguilar CN. Ellagic acid: Biological properties and biotechnological
development for production processes. African J Biotechnol. 2011;10:4518-4523.
- Tzulker R, Glazer I, Bar-Ilan I, Holland D, Aviram M, Amir
R. Antioxidant activity, polyphenol content, and related compounds in different
fruit juices and homogenates prepared from 29 different pomegranate accessions.
J Agric Food Chem. 2007;55:9559-9570.
- Lei F, Xing D-M, Xiang L, et al. Pharmacokinetic study of
ellagic acid in rat after oral administration of pomegranate leaf extract. J
Chromatogr B. 2003;796:189-194.
- Seeram NP, Henning SM, Zhang Y, Suchard M, Li Z, Heber D.
Pomegranate juice ellagitannin metabolites are present in human plasma and some
persist in urine for up to 48 h. J Nutr. 2006;136:2481-2485.
- Ben Nasr C, Ayed N, Metche M. Quantitative determination of
the polyphenolic content of pomegranate peel. Z Lebensm Unters Forsch.
1996;203:374-378.
- Zhou B, Wu Z, Li X, Zhang J, Hu X. Analysis of ellagic acid
in pomegranate rinds by capillary electrophoresis and high-performance liquid
chromatography. Phytochem Anal. 2008;19:86-89.
- Panichayupakarananta P, Issuriya A, Sirikatitham A, Wang W.
Antioxidant assay-guided purification and LC determination of ellagic acid in
pomegranate peel. J Chromatogr Sci. 2010;48:456-459.
- Fischer UA, Carle R, Kammerer DR. Identification and
quantification of phenolic compounds from pomegranate (Punica granatum L.)
peel, mesocarp, aril and differently produced juices by HPLC-DAD–ESI/MSn. Food
Chem. 2011;127:807-821.
- Jimenez Del Rio M, Ramazanov A, Sikorski S, Ramazanov Z,
Chkhikvishvili I. A new method of standardization of health-promoting
pomegranate fruit (Punica granatum) extract. Georgian Medical News.
2006;140:70-77.
- Calín-Sánchez Á, Figiel A, Hernández F, Melgarejo P, Lech K,
Carbonell-Barrachina ÁA. Chemical composition, antioxidant capacity, and
sensory quality of pomegranate (Punica granatum L.) arils and rind as affected
by drying method. Food Proc Biotechnol. 2013;6:1644-1654.
- Gómez-Caravaca M, Verardo V, Toselli M, Segura-Carretero A,
Fernández-Gutiérrez A, Caboni MF. Determination of the major phenolic compounds
in pomegranate juices by HPLC-DAD-ESI-MS. J Agric Food Chem. 2013;61:5328-5337.
- Seeram NP, Lee R, Heber D. Bioavailability of ellagic acid
in human plasma after consumption of ellagitannins from pomegranate (Punica
granatum L.) juice. Clin Chim Acta. 2004;348:63-68.
- Stoner GD, Sardo C, Apseloff G, et al. Pharmacokinetics of
anthocyanins and ellagic acid in healthy volunteers fed freeze-dried black
raspberries daily for 7 days. J Clin Pharmacol. 2005;45:1153-1164.
- Mertens-Talcott SU, Jilma-Stohlawetz P, Rios J, Hingorani L,
Derendorf H. Absorption, metabolism, and antioxidant effects of pomegranate (Punica
granatum L.) polyphenols after ingestion of a standardized extract in healthy
human volunteers. J Agric Food Chem. 2006;54:8956-8961.
- Seeram NP, Zhang Y, McKeever R, et al. Pomegranate juice and
extracts provide similar levels of plasma and urinary ellagitannin metabolites
in human subjects. J Med Food. 2008;11:390-394.
- Beserra AMSS, Souza MdC, Colodel EM, et al. Toxicological
evaluation of ellagic acid in rodents. Rev Bras Farm. 2010;91:16-24.
- Tasaki M, Umemura T, Maeda M, et al. Safety assessment
of ellagic acid, a food additive, in a subchronic toxicity study using F344
rats. Food Chem Toxicol. 2008;46:1119-1
|