Issue:
122
Page: 6-16
Tongkat Ali
Eurycoma longifolia
Family: Simaroubaceae
by Josef Brinckmann, Thomas Brendler
HerbalGram.
2019; American Botanical Council
INTRODUCTION
Eurycoma longifolia is a slow-growing evergreen plant that belongs to the quassia family (Simaroubaceae) of shrubs and trees, which includes 19 or 20 genera and more than 100 species with pantropical distributions.1-3 There are only three known Eurycoma species and one subspecies2: E. apiculata, distributed in Peninsular Malaysia and Sumatra (Indonesia)4; E. harmandiana, which occurs in the border regions between Thailand and Laos5,6; E. longifolia, native to parts of southeastern Asia (Indonesia, Malaysia, Brunei Darussalam, Singapore, Thailand, Cambodia, Laos, and Vietnam)7; and E. longifolia subsp. eglandulosa, endemic to the Philippines.8 It is difficult to differentiate E. longifolia from E. apiculata, though the latter typically has slightly bigger leaves9 and usually short inflorescences or infructescences that point upward, whereas the fertile parts of E. longifolia are long and drooping.10 Some Vietnamese compendia list Crassula pinnata (Crassulaceae) as a synonym of E. longifolia,11 but The Plant List classifies C. pinnata as an unresolved name.2
The E. longifolia tree is dioecious (male and female flowers occur on separate plants) with hairy, purplish-crimson, bell-shaped flowers.12 The tree fruits after the second or third year, completes maturation at about 25 years, and can reach 15 to 18 m (49 to 59 ft) in height.13 It occurs in the understory of primary and secondary forests of Indonesia,14 mainly Sumatra Island and Kalimantan of Borneo Island,15 including coal mine reclamation areas within biodiversity hotspots16; in beach forests on sandy soil as understory treelets in Malaysia12; and in endangered forests of the Indo-Burma biodiversity hotspot17 (a region that has lost about 95% of its original natural habitat18), including parts of Cambodia,19 and in the understory of evergreen and mixed deciduous forests in Thailand.19,20
While E. longifolia is known as pasak bumi in Indonesia, tongkat ali in Malaysia, and bách bệnh in Vietnam, among other names,21 this article will use “tongkat ali,” which is the name most often encountered in the US market. A standard common name for this species is not provided in the American Herbal Products Association’s Herbs of Commerce, 2nd edition.22 While some companies in Malaysia use the marketing name “Malaysian ginseng,” the term “ginseng” is narrowly defined in US regulation (7 CFR §65.145 Ginseng) as ginseng root of the genus Panax.23 As per US Code (21 USC §343(u) Ginseng), no herb or herbal ingredient may be labeled or marketed in the United States as “ginseng” unless it is derived from Panax species.24
Until recently, almost all of the commercial supply of tongkat ali was obtained from wild collection in forests of Indonesia, Malaysia, and Vietnam. In the early 2000s, commercial plantations were started in Malaysia.25
HISTORY AND CULTURAL SIGNIFICANCE
In 1822, Scottish botanist and medical practitioner William Jack (1795-1822), while employed as a surgeon with the East India Company, assigned the Latin name Eurycoma longifolia in volume II of Malayan Miscellanies.26 The genus name Eurycoma stems from the Greek eurys meaning “large” and kome meaning “tuft of hairs,”27 referring to its compound leaves that spiral out at the tip of its slender trunk in a large, dense rosette.28 Jack described the small tree having leaves that are two feet long, thus the species name longifolia. In Latin, longus means “long” and folia means “leaf.” Jack’s 1822 monograph did not use the common name tongkat ali (which means “Ali’s root”) but listed a Malay name for the plant, kayu kabal, and stated that the tree is found in Singapore (at that time a British colony established in 1819) and in Tapanuli and Bencoolen (at that time possessions of the British Empire in Sumatra; now part of Indonesia).26
The cultural history of coffee (Coffea arabica, Rubiaceae) in Southeast Asia has intersected with that of tongkat ali. In the late 18th century, cultivated coffee ranked among the most important commodities for the colonial government in Tapanuli. British merchant ships loaded coffee at the port of Tapanuli until the trade was taken over by Dutch colonials in 1833.29 Today, tongkat ali-based coffee and tea (Camellia sinensis, Theaceae) products are among the most common beverage combinations in the region.30 Recently, analytical methods have been developed to identify tongkat ali extract when combined with coffee.31 A novel food application was made in 2016, awaiting decision at the time of this writing, seeking approval for the use of tongkat ali root extract as a component of coffee beverages in the European Union (EU).32
In Brunei Darussalam and Singapore, preparations of E. longifolia (root, root bark, and/or leaf) are used for lowering blood pressure.25 In Cambodian traditional medicine, the roots are used as an antidote against intoxication, and, when boiled or soaked in wine before drinking, to enhance human power. The Batak people of North Sumatra prepare a decoction of the roots that is used to enhance stamina and treat stomachache, fever, and malaria.33 Men of the Kutai ethnic group in East Kalimantan, Indonesia, drink an infusion of the tap roots for backaches and an aphrodisiac effect.34 For hundreds of years, traditional medicine practitioners of the isolated Lingga Malay people — a lineage descending from the former Riau-Lingga Sultanate, a monarchy that was recognized by the British and Dutch colonial empires — have protected their traditional formulations including obat pahit, which means “bitter medicine.” Prepared as an aqueous decoction and taken as a “body energy keeper,” obat pahit tea may contain up to 24 herbs, including roots of E. longifolia, Bauhinia semibifida (Fabaceae), Cnestis palala (Connaraceae), and Rhodomyrtus tomentosa (Myrtaceae), among others.35
In Malay medicine, tongkat ali roots are used for fever, boils, wounds, ulcers, syphilis, bleeding gums, and as medication after birth.14 The bark is used as a vermifuge.7 The Semelai people (population ca. 2,000) of Tasek Bera in Peninsular Malaysia prepare aqueous decoctions of tongkat ali root in combination with roots of Iguanura wallichiana (Arecaceae) and Calamus insignis (Arecaceae) as an aphrodisiac or to boost energy. Tongkat ali root is also decocted with whole plant of Smilax calophylla (Smilacaceae) or tuber of S. myosotiflora for similar purposes.36 Eurycoma longifolia is among the most used plant species of the Temuan people, indigenous to parts of western Peninsular Malaysia, who prepare an aqueous decoction of the root that is taken orally to treat muscle pain and conditions related to diabetes and hypertension.37 In Thai traditional medicine, the roots are used to treat sore throat and tonsillitis, for detoxification, and for their antipyretic, expectorant, anti-tuberculosis, anthelmintic, diaphoretic, and antimalarial actions. The bark is used as an antipyretic and antimalarial.14 In Vietnam, the very bitter bark traditionally is used for indigestion.7
The first quality standards monograph for tongkat ali root appeared in volume I of the Malaysian Herbal Monograph in 1999,38 which was significantly revised and updated in the currently valid 2015 edition.39 Based in part on the Malaysian Herbal Monograph, the Department of Standards Malaysia published a specification developed by the Working Group on Phytopharmaceutical Aspect of Herbs for a freeze-dried aqueous extract of tongkat ali root.40 In 2001, the Indonesian Institute of Sciences, Research Center for Chemistry published an initial monograph for quality control testing.41 The Indonesian National Agency of Drug and Food Control included monographs (for pasak bumi) in the Acuan Sediaan Herbal (“Manual of Herbal Drug Preparations”) volumes VI (2011)42 and VII (2012).43 In 2013, a monograph for the extract was included in volume II of Pedoman Teknologi Formulasi Sediaan Berbasis Ekstrak (“Formulation Technology Guidelines for Herbal Drug Extract Preparations”).44 Despite these monographs, standardization of extracts and analytical testing has been hampered by the very high cost of the reference standard eurycomanone, and alternative methods are in development.45-47
CURRENT AUTHORIZED USES IN COSMETICS, FOODS, AND MEDICINES
In Vietnam, E. longifolia (bách bệnh) is included on the Ministry of Health’s positive list of traditional herbal medicinal tonic drugs that are covered under the national insurance health fund.48 In Malaysia, at the time of this writing, there are 56 registered traditional medicine products that list E. longifolia as an active ingredient. Most of the listings appear to be male enhancement drugs.49 In volume VI of the Indonesian manual of herbal drug preparations, the extract of the root (ekstrak pasak bumi) is listed as an anticancer drug with a prescribed dosage of 300 mg, twice daily, with a maximum dosage of 1,000 mg daily.42 Volume VII of the manual includes monographs for uses as a male infertility drug and as a male aphrodisiac drug.43 For specific treatment of erectile dysfunction, Indonesia’s new national formulary of traditional herbal medicines prescribes one 400-mg capsule of extract daily.50
In the United States, tongkat ali may be used as a component of dietary supplement products, which require notification with the US Food and Drug Administration (FDA) within 30 days of marketing if a structure-function claim is made.51 In Canada, tongkat ali is regulated as an active ingredient of licensed natural health products (NHPs), which require pre-marketing authorization from the Natural and Non-prescription Health Products Directorate (NNHPD). At the time of this writing, there are 206 licensed NHPs in Canada that list E. longifolia as an active ingredient.52 In the EU, there are some authorized applications for use of an extract of the root in cosmetic products, specifically for skin conditioning and skin protecting functions.53 However, for oral ingestion, E. longifolia is presently classified as an unauthorized novel food in the EU. There has been a request for a determination as to whether it requires authorization under the Novel Food Regulation. According to EU regulatory authorities, E. longifolia was not used as a food or food ingredient in the EU before May 15, 1997, and, therefore, a pre-marketing safety assessment under the Novel Food Regulation is required.54
MODERN RESEARCH
The health benefits and traditional uses of tongkat ali root extract, namely its antimalarial, anticancer, antidiabetic, aphrodisiac, proandrogenic, and antimicrobial effects, have been confirmed in a wide range of investigations: laboratory research, animal models, and human clinical trials.55-61 Tongkat ali’s adaptogen-like phytoandrogenic properties make it a promising remedy to address a wide range of male sexual health-related ailments, from erectile dysfunction62 to age-related loss of virility59 and osteoporosis.63 It may also provide a natural alternative to testosterone replacement therapy.64
Various chemical compounds have been isolated and characterized from the root, leaf, and stem including β-carboline alkaloids, such as canthin-6-one; squalene derivatives; triterpenes; biphenyl neolignans; and quassinoids, specifically lonilactone, eurycomanone, 13α(21)-epoxyeurycomanone, eurycomanol, eurycomalide A and B, eurycolactone, laurycolactone, and eurycomalactone.55,65,66 The pharmacokinetics of tongkat ali have been addressed in a number of reviews.56,30 There is very little evidence that tongkat ali interacts with conventional pharmaceutical drugs; most drug-metabolizing cytochrome P450 enzyme isoforms are not affected (up- or downregulated) by tongkat ali administration, making it a suitable adjuvant with low interaction potential in the treatment of various disease states.67
The major quassinoids in tongkat ali appear to be responsible for stimulating the release of free testosterone from its binding proteins and improving overall hormone profiles. The quassinoid eurycomanone also has been shown to stimulate spermatogenesis.68 Recently, a number of compounds isolated from tongkat ali have demonstrated promising antiproliferative and cytotoxic effects, the latter by inducing apoptosis by up-regulating p53 (a tumor suppression protein) and Bax (a pro-apoptotic protein) and down-regulating Bcl-2 (an anti-apoptotic protein) expression.65,69 Based on previous investigations (e.g., Varghese et al, 2013),70 a number of NF-κB inhibitors responsible for tongkat ali’s anti-inflammatory properties have been identified.71
Overall, tongkat ali has been shown to possess an excellent safety profile. Using rat models, scientists have investigated the oral toxicity of the branded tongkat ali root aqueous dry extract Physta® (Biotropics Malaysia Berhad; Shah Alam, Malaysia), which is also known as LJ100™ in the United States (distributed by HP Ingredients; Bradenton, Florida). Based on these experiments, researchers reported a median lethal dose (LD50) of more than 2,000 mg/kg body weight (acute) and a no-observed-adverse-effect-level (NOAEL) greater than 1,000 mg/kg body weight (sub-acute).72,73 The genotoxicity potentials of tongkat ali72 and Physta74 also have been investigated. No mutagenic, clastogenic, or histopathological changes were observed. Physta was not toxic to Salmonella strains at doses up to 5 mg/plate, and it did not alter relative polychromatic erythrocytes (PCEs) or increase incidence of micronucleated PCEs in a mouse peripheral blood cell micronucleus assay.74
Effectively all of the studies listed in Table 1 were conducted with Physta, which is manufactured using a patented hot-water extraction process. The studies are highly heterogeneous in power and quality. Results of early investigations were presented as papers or posters at international conferences and have never been fully published. This makes it almost impossible to fully describe, let alone evaluate, their endpoints, methodologies, and results. It is likely that low dosage and/or short duration of supplementation led to failure in demonstrating beneficial effects in some of the studies,75,76 the exception being the results published by Chen et al (2014).77 Nonetheless, when considering the substantial body of traditional use and pre-clinical data, a positive benefit-risk ratio can be derived at least for the commercial product Physta, which has demonstrated an excellent safety profile in human trials, with the latest being George et al (2018),78 which evaluated a combination of Physta and multivitamins. None of the studies reported adverse events linked to the use of the product. 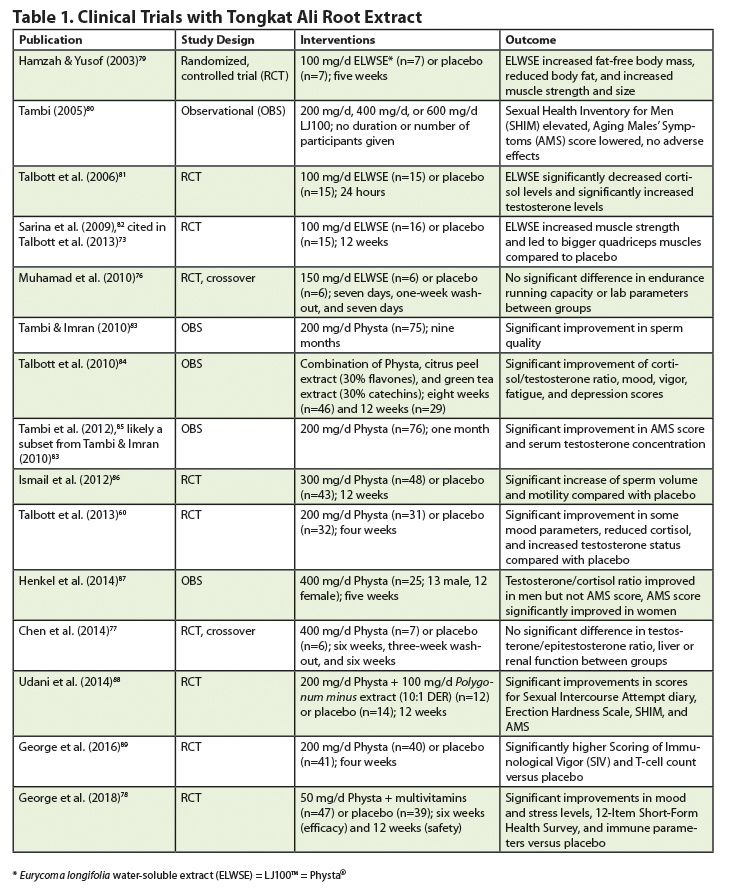
ADULTERATION
Adulteration of tongkat ali products with synthetic pharmaceutical drug substances has been documented since the early 2000s and remains a serious concern, with potential impacts on human health and safety. In 2004, the Drug Control Authority of Malaysia issued a warning about the presence of the erectile dysfunction drug tadalafil in a product called Shitek Tongkat Ali Plus, which had a fraudulent marketing authorization number on the label.90 In a separate case in 2012, tadalafil was detected in the gelatin capsules that the tongkat ali extract was filled into, but not in the extract itself. It was theorized that the adulteration was likely accomplished by adding tadalafil powder to gelatin during the manufacturing of the capsules.91 In a 2015 study, researchers used a high-performance thin-layer chromatography (HPTLC) method to screen for the presence of three phosphodiesterase type 5 inhibitors (sildenafil, vardenafil, and tadalafil) and eight analogs in finished products. Of the 45 products screened, 31 were found to contain at least one of these compounds, including a product labeled as Tongkat Ali Power Plus (tablets) that tested positive for sildenafil.92
One study asserted that, due to the presence of bitter-tasting quassinoids, tongkat ali extracts that do not have a distinct bitter taste may likely be adulterated, with some containing no tongkat ali root extract at all, but rather tadalafil, sildenafil, or vardenafil.60 This could also be considered economic adulteration because the erectile dysfunction active ingredients cost less than authentic extracts of tongkat ali root. A 2018 study, using DNA barcoding validated by high-performance liquid chromatography (HPLC) analysis, reported that only 37% of 11 sampled products (nine capsules, one tea, and one tablet) labeled as containing E. longifolia, purchased from retail shops in four different areas of Malaysia, were authentic.93 However, the study compared the products in capsule, tea, or tablet form against an extract prepared from authenticated tongkat ali root. It was not stated whether the dosage of the extract or powder in the finished products took into consideration the dilution factor introduced by excipients or other ingredients.
In the United States, there have been several recalls of instant tongkat ali-coffee beverages including Kopi Jantan Tradisional Natural Herbs Coffee (Bestherbs Coffee; Grand Prairie, Texas) and Stiff Bull Herbal Coffee, due to the FDA’s detection of desmethyl carbodenafil (a compound similar to sildenafil) in the products, and Caverflo Natural Herbal Coffee, due to FDA laboratory analysis confirming the presence of both sildenafil and tadalafil.94,95 Furthermore, despite the Malaysian Ministry of Health’s having banned kopi jantan (“male coffee”) products spiked with erectile dysfunction drugs, brands like Kopi Jantan Tradisional, Kopi Tenaga Tok Lebai Plus, and Kopi Panggung Al-Ambiak remain popular and openly available in markets. The content of tongkat ali per serving is rarely stated on the labels of such products, which likely contain very low amounts of powdered root, not extract. In contrast, there are functional coffee products available that are labeled to contain 50 mg of clinically tested standardized extract per sachet (i.e., Nu-caffe from Biotropics Malaysia).
Due to shared or similar common names, inadvertent substitution may also occur. In Malaysia, other substances share the common name tongkat ali, some of which are also used for similar purposes, such as the fungus Entomophthora apiculata (Entomophthorales) and the roots of Stemona tuberosa (Stemonaceae),96 Polyalthia bullata (Annonaceae), and Goniothalamus spp. (Annonaceae).30
SUSTAINABILITY AND FUTURE OUTLOOK
In the Malaysian state of Sarawak on Borneo Island, E. longifolia, known locally as sengkayap, became a protected plant in 1998 under Sarawak’s Wild Life Protection Ordinance.97,98 In 2003, E. longifolia was included in the list of medicinal genetic resources of Laos to be managed and protected as per the Prime Minister’s decree on natural resources for medicines.99 Known as linátog in the Tagalog language, E. longifolia subsp. eglandulosa is classified as an endangered species on the National List of Threatened Philippine Plants.100
Conservation and sustainability of the tongkat ali tree have been a concern for decades. As it is mainly the roots that are used, destructive harvesting by uprooting the entire tree was common. By the late 1990s, when overharvesting of wild populations became apparent, the Malaysian Ministry of Primary Industries formed a task force, taking actions to prevent extinction and promote cultivation.101 Although the tree itself reaches maturity in about 25 years,13 roots are generally harvested from trees of seven to 10 years’ maturity. Nonetheless, Malaysia’s National Forestry Council (NFC) began to promote development of the country’s medicinal plant sector, including the popular, high-value tongkat ali. In this context of trade promotion for the root of a slow-growing tree, the Forest Research Institute Malaysia (FRIM) was tasked with shortening the root maturity period from seven years to five years.102
Some Malaysian institutions have taken steps to manage, conserve, and determine sustainable use of wild tongkat ali populations. The Malaysian Agricultural Research and Development Institute (MARDI), for example, has implemented projects to ensure that maximum genetic diversity within the current wild population will be preserved and managed for future needs and the formation of cluster farms under the East Coast Economic Region of Malaysia for replanting.9 The agronomical studies conducted by FRIM and genetic material conservation by MARDI provided resources to help enable successful tongkat ali cultivation and future sustainability. Replanting of deforested areas has been undertaken successfully in Sabah, Malaysia, with detailed forest management systems in place, which can provide suitable habitat for tongkat ali trees.103 Additionally, in 2012, the first successful adventitious root induction for tongkat ali was reported, paving the way for potential mass production of root cultures in a bioreactor system.104 (Adventitious roots are roots that arise from any nonroot plant tissue.) The Malaysian Nuclear Agency claims to have developed an advanced bioreactor technology facility, designed to mass produce root cultures that, they say, will be able to reduce the tongkat ali root maturing time from 7-10 years down to mere months.105 In addition, pilot projects involving tongkat ali seed germination, seedling nurseries, and replanting in the forest have been initiated with indigenous communities to support sustainability (Annie George, senior manager at Biotropics Malaysia, email to T. Smith, April 5, 2019).
Indonesia and Malaysia are the main exporters of tongkat ali ingredients and products.41 The species also ranks among the most important high-demand medicinal plants wild-collected from forests in Vietnam.106 Tongkat ali is the highest volume and value wild-collected medicinal plant in Malaysia.41 A 2001 supply and demand study estimated that about 21,000 kg were wild harvested in Malaysia annually and that domestic annual demand was about 54,189 kg by Malay traditional industries.107 According to FRIM, in 2009, the sustainably produced supply of tongkat ali roots was estimated at about 100 tons (about 90,718 kg) annually. At that time, the government held 167 ha (413 acres) of tongkat ali tree plantations in Peninsular Malaysia, while the private sector operated 29 ha (72 acres), with a yield of about four tons (about 3,629 kg) of roots per ha.108 As a result, the government of Malaysia has banned the export of viable tongkat ali raw material (roots) to prevent use of its plant genetic resources without prior informed consent.109
In 2010, the Malaysian Ministry of Health projected an annual 15% increase in tongkat ali demand and valued the overall tongkat ali market at 7 billion MYR (Malaysian Ringgits), or approximately $1.7 billion.110 In Indonesia, extraordinarily high market prices continue to encourage increased exploitation of wild E. longifolia trees. In 2014, the export price for chipped root was reported to be nearly $250 per kg (about $550 per lb), and prices of extracts ranged from about $1,625 to $3,243 per kg (about $3,582 to $7,149 per lb), depending on the drug-to-extract ratio, compared to a price range of $375 to $900 per kg (about $827 to $1,984 per lb) for the same extracts in 2012, a tripling to quadrupling of extract prices in just two years. This increase appears to be related to the increasing scarcity of wild resources and increasing demand.18
Like many medicinal plants that have unique chemical compositions and corresponding pharmacological actions and that developed in a biodiverse forest ecosystem, future access may depend on protection and conservation of biodiversity hotspots. Efforts to conserve the genetic diversity of this species are underway in some parts of its range. Continuing to explore innovative methods for mass production of plant and root cultures is also important, and, in the future, may result in tongkat ali preparations of quality and effect comparable to those of mature wild tree roots.
References
- Alves IABS, Miranda HM, Soares LAL, Randau KP. Simaroubaceae family: Botany, chemical composition and biological activities. Rev Bras Farmacogn. 2014;24(4):481-501.
- Eurycoma longifolia. The Plant List (2013). Version 1.1. Available at: http://theplantlist.org/tpl1.1/record/kew-2805148. Accessed April 29, 2019.
- Hua P, Thomas WW. Simaroubaceae. ku mu ke. In: Flora of China: Oxalidaceae through Aceraceae. Vol 11. St. Louis, MO: Missouri Botanical Garden Press; 2008:100-104.
- Hassler M. World Plants: Synonymic Checklists of the Vascular Plants of the World (version April 2018). In: Roskov Y, Ower G, Orrell T, et al., eds. Species 2000 & ITIS Catalogue of Life, 24th December 2018. Digital resource at www.catalogueoflife.org/col. Leiden, the Netherlands: Species 2000: Naturalis; 2019.
- Kanchanapoom T, Kasai R, Chumsri P, Yamasaki K. Quassinoids from Eurycoma harmandiana. Phytochemistry. 2001;57(8):1205-1208.
- Sydara K, Xayvue M, Souliya O, Elkington BG, Soejarto DD. Inventory of medicinal plants of the Lao People’s Democratic Republic: A mini review. J Med Plants Res. 2014;8(43):1262-1274.
- Uphof JCT. Dictionary of Economic Plants. 2nd ed. New York, NY: Verlag von J. Cramer; 1968.
- Lillo EP, Fernando ES, Lillo MJR. Plant diversity and structure of forest habitat types on Dinagat Island, Philippines. J Asia Pac Biodivers. 2018.
- Nordin MS. Distribution of the population of tongkat ali (Eurycoma spp.) in Malaysia based on data taken from herbarium records. Medicinal and Aromatic Plants. 2014;3(2):155.
- Tan AL, Nurnida MK, Tan HP. Notes on the morphological characteristics of Eurycoma spp. observed and its status in Peninsular Malaysia. In: Program Book. Contributions of Flora Malesiana to the Welfare of People in Asia. Bogor, Indonesia: 9th International Flora Malesiana Symposium; 2013:233-234.
- Đỗ TL. Những cây thuốc và vị thuốc Việt Nam. Hà Nội, Việt Nam: Nhà Xuất Bản Y Học; 2004.
- Chan LK, Su TS, Teo CKH. A preliminary study on the germination of Eurycoma longifolia Jack (tongkat ali) seeds. Pertanika J Trop Agric Sci. 2002;25(1):27-34.
- Elhag HEEA, Sulaiman AZ, Azilah A. A Review on the Exraction Methods of Extracts and Phytochemicals from Eurycoma longifolia (Tongkat Ali Jack). In: Ibrahim Z, Pebrianti D, Mohamed N, Ghani SA, Geem CH, eds. Proceedings of The National Conference for Postgraduate Research (NCON-PGR 2016). Pekan, Pahang: Universiti Malaysia Pahang (UMP); 2016:253-259.
- Ali RM, Samah ZA, Mustapha NM, Hussein N. ASEAN Herbal and Medicinal Plants. Jakarta, Indonesia: ASEAN Secretariat; 2010.
- McCann G, Hsu YC. Animism and Traditional Knowledge Disappear in Virachey National Park, Cambodia. In: Verschuuren B, Furuta N, eds. Asian Sacred Natural Sites. Philosophy and Practice in Protected Areas and Conservation. London, UK: Routledge; 2016.
- Tordoff A, Baltzer M, Fellowes J, Pilgrim J, Langhammer P. Key biodiversity areas in the Indo-Burma Hotspot: Process, progress and future directions. J Threat Taxa. 2012;4(8):2779-2787.
- Hamidah S, Arifin YF, Fitriani A. Micro climate assessment of medicinal plant habitat for the first step of domestication. Acad Res Int. 2018;9(3):145-150.
- Kartikawati SM, Zuhud EAM, Hikmat A, Kartodihardjo H, Fuadi M. Habitat preferences, distribution pattern, and root weight estimation of Pasak Bumi (Eurycoma longifolia Jack). J Man Hut Trop. 2014;20(1):43-50.
- Trimanto T, Sofiah S. Exploration of flora diversity and recommending species for reclamation of coal mining with biodiversity concept in Besiq Bermai Forest, East Borneo. J Trop Life Sci. 2018;8(2):97-107.
- Choon KK, ed. Forest Ecology Study Final Technical Report. Bangkok, Thailand: Forestry Research Center, Faculty of Forestry, Kasetsart University; 2004.
- Tnah LH, Lee CT, Lee SL, Ng KKS, Ng CH, Hwang SS. Microsatellite markers of an important medicinal plant, Eurycoma longifolia (Simaroubaceae), for DNA profiling. Am J Bot. 2011;98(5):e130-e132.
- McGuffin M, Kartesz JT, Leung AY, Tucker AO. American Herbal Products Association’s Herbs of Commerce. 2nd ed. Silver Spring, MD: American Herbal Products Association; 2000.
- United States Department of Agriculture. 7 CFR § 65.145 - Ginseng. In: Code of Federal Regulations. Washington, DC: US Government Printing Office; 2018.
- Office of the Law Revision Counsel. 21 USC 343: Misbranded food. In: United States Code. Washington, DC: US House of Representatives; 2018.
- Handa SS, Rakesh DD, Vasisht K. Compendium of Medicinal and Aromatic Plants - ASIA. Vol II. Trieste, Italy: United Nations Industrial Development Organization and the International Centre for Science and High Technology (ICS-UNIDO); 2006.
- Jack W. Descriptions of Malayan plants. In: Malayan Miscellanies. Vol II. Bencoolen: Sumatran Mission Press; 1822.
- Craig J. A New Universal Etymological Technological, and Pronouncing Dictionary of the English Language. Vol I. London: Henry George Collins; 1848.
- Forest Research Institute Malaysia. The rare sight of fruiting Tongkat Ali. 2014. Available at: www.frim.gov.my/the-rare-sight-of-fruiting-tongkat-ali/. Accessed April 29, 2019.
- Agustono B, Junaidi. The Dutch colonial economic policy: Coffee exploitation in Tapanuli residency, 1849–1928. KEMANUSIAAN Asian J Humanit. 2018;25(2):49–71.
- Rehman SU, Choe K, Yoo HH. Review on a traditional herbal medicine, Eurycoma longifolia Jack (tongkat ali): Its traditional uses, chemistry, evidence-based pharmacology and toxicology. Molecules. 2016;21(3):331.
- Fadzil NF, Wagiran A, Mohd Salleh F, Abdullah S, Mohd Izham NH. Authenticity testing and detection of Eurycoma longifolia in commercial herbal products using bar-high resolution melting analysis. Genes. 2018;9(8):408.
- Biotropics Malaysia Berhad. Application for the Approval of Tongkat Ali Root Extract as a Novel Food. Selangor, Malaysia: Biotropics Malaysia Berhad; 2016.
- Silalahi M, Nisyawati. Etnobotani pasak bumi (Eurycoma longifolia) pada etnis Batak, Sumatera Utara. Pros Sem Nas Masy Biodiv Indon. 2015;1(4):743-746.
- Saragih B. Economic value of non-timber forest products among Paser Indigenous People of East Kalimantan, Universiteit Leiden; 2011.
- Fitmawati, Sofiyanti N, Roza RM, et al. Traditional medicinal formulation: Obat pahit from Lingga Malay ethnic in Riau Archipelago, Indonesia. Biodiversitas. 2017;18(3):1196-1200.
- Wetlands International. Living Pharmacies in Tasek Bera. A Wetland of International Importance in Malaysia. Wageningen, The Netherlands: Wetlands International; 2006.
- Azliza MA, Ong HC, Vikineswary S, Noorlidah A, Haron NW. Ethno-medicinal resources used by the Temuan in Ulu Kuang Village. Stud Ethno-Med. 2012;6(1):17-22.
- Ismail Z, Ismail N, Lassa J. Malaysian Herbal Monograph. Vol I. Kuala Lumpur, Malaysia: Malaysian Monograph Committee; 1999.
- Malaysian Herbal Monograph Committee. Malaysian Herbal Monograph 2015. Kuala Lumpur, Malaysia: Institute for Medical Research; 2015.
- Department of Standards Malaysia. Phytopharmaceutical Aspect of Freeze Dried Water Extract from Tongkat Ali Roots - Specfication (Malaysian Standard MS 2409:2011 / ICS: 67-040). Cyberjaya, Malaysia: Department of Standards Malaysia, Ministry of Science, Technology and Innovation; 2011.
- Brinckmann JA. Market for Malaysian Natural Ingredients used in Cosmetic, Dietary Supplement and Pharmaceutical Products. Putrajaya, Malaysia: Malaysian Herbal Corporation; 2007.
- Direktorat Obat Asli Indonesia. Acuan Sediaan Herbal. Vol 6. 1 ed. Jakarta, Indonesia: Badan Pengawas Obat dan Makanan RI; 2011.
- Direktorat Obat Asli Indonesia. Acuan Sediaan Herbal. Vol 7. 1 ed. Jakarta, Indonesia: Badan Pengawas Obat dan Makanan RI; 2012.
- Badan POM RI (National Agency of Drug and Food Control of Republic of Indonesia). Pedoman Teknologi Formulasi Sediaan Berbasis Ekstrak. Vol 2. Jakarta, Indonesia: Direktorat Obat Asli Indonesia, Deputi Bidang Pengawas Obat Tradisional, Kosmetik Dan Produk Komplemen; 2013.
- Khari N, Aisha AFA, Ismail Z. Reverse phase high performance liquid chromatography for the quantification of eurycomanone in Eurycoma longifolia Jack (Simaroubaceae) extracts and their commercial products. Trop J Pharm Res. 2014;13(5):801-807.
- Zaini NN, Osman R, Juahir H, Saim N. Development of chromatographic fingerprints of Eurycoma longifolia (Tongkat Ali) roots using online solid phase extraction-liquid chromatography (SPE-LC). Molecules. 2016;21(5):583.
- Yunianto P, Nurhadi N, Supriyono A. Isolasi, validasi metode dan optimasi awal proses ekstraksi senyawa penanda eurycomanon dari akar tanaman pasak bumi (Eurycoma longifolia). Chim Nat Acta. 2017;5(2):70-76.
- Bộ Y tế Việt Nam. VĂN BẢN PHÁP LUẬT KHÁC - VĂN BẢN HỢP NHẤT - THÔNG TƯ: Ban hành Danh mục thuốc đông y, thuốc từ dược liệu và vị thuốc y học cổ truyền thuộc phạm vi thanh toán của quỹ bảo hiểm y tế. Hà Nội, Việt Nam: Bộ Y tế, Cộng hòa xã hội chủ nghĩa Việt Nam; 2018.
- National Pharmaceutical Regulatory Agency. Sistem Pendaftaran Produk & Perlesenan QUEST 3+. Selangor, Malaysia: National Pharmaceutical Regulatory Agency, Ministry of Health Malaysia; 2019.
- Menteri Kesehatan Republik Indonesia. Peraturan Menteri Kesehatan Tentang Formularium Obat Herbal Asli Indonesia. Jakarta, Indonesia: Menteri Kesehatan Republik Indonesia; 2016.
- US Food and Drug Administration. Regulations on Statements Made for Dietary Supplements Concerning the Effect of the Product on the Structure or Function of the Body; Final Rule. Federal Register. 2000;65(4):1000-1050.
- Natural and Non-prescription Health Products Directorate. Licensed Natural Health Products Database (LNHPD). Ottawa, ON: Health Canada; 2019.
- European Commission. Cosmetic ingredient (CosIng) database. Brussels, Beglium: DG Internal Market, Industry, Entrepreneurship and SMEs; 2019.
- European Commission. Novel Food Catalogue. Brussels, Belgium: European Commission; 2019.
- Bhat R, Karim AA. Tongkat ali (Eurycoma longifolia Jack): A review on its ethnobotany and pharmacological importance. Fitoterapia. 2010;81(7):669-679.
- Norhidayah Mohamed A, Vejayan J, Yusoff M. Review on Eurycoma longifolia pharmacological and phytochemical properties. J Appl Sci. 2015;15(6):831-844.
- Han YM, Woo S-U, Choi MS, et al. Antiinflammatory and analgesic effects of Eurycoma longifolia extracts. Arch Pharm Res. 2016;39(3):421-428.
- Lim TK. Edible Medicinal and Non-Medicinal Plants: Volume 11 Modified Stems, Roots, Bulbs. 1st ed. Switzerland: Springer International Publishing; 2016.
- Thu HE, Mohamed IN, Hussain Z, Jayusman PA, Shuid AN. Eurycoma longifolia as a potential adoptogen of male sexual health: a systematic review on clinical studies. Chin J Nat Med. 2017;15(1):71-80.
- Talbott SM, Talbott JA, George A, Pugh M. Effect of tongkat ali on stress hormones and psychological mood state in moderately stressed subjects. J Int Soc Sport Nutr. 2013;10(1):28.
- Thu HE, Hussain Z, Mohamed IN, Shuid AN. Recent advances in antibacterial, antiprotozoal and antifungal trends of Eurycoma longifolia: A review of therapeutic implications and future prospects. Curr Drug Targ. 2018;19(14):1657-1671.
- Kotirum S, Ismail SB, Chaiyakunapruk N. Efficacy of tongkat ali (Eurycoma longifolia) on erectile function improvement: Systematic review and meta-analysis of randomized controlled trials. Complement Ther Med. 2015;23(5):693-698.
- Effendy NM, Mohamed N, Muhammad N, Naina Mohamad I, Shuid AN. Eurycoma longifolia: Medicinal plant in the prevention and treatment of male osteoporosis due to androgen deficiency. Evid Based Complement Alternat Med. 2012;2012:9.
- George A, Henkel R. Phytoandrogenic properties of Eurycoma longifolia as natural alternative to testosterone replacement therapy. Andrologia. 2014;46(7):708-721.
- Kuo P-C, Damu AG, Lee K-H, Wu T-S. Cytotoxic and antimalarial constituents from the roots of Eurycoma longifolia. Bioorg Med Chem. 2004;12(3):537-544.
- Zakaria N, Mohd K, Hamil MSR, Memon DAH, Asmawi M, Ismail Z. Characterization of primary and secondary metabolites of leaf and stem extracts from Eurycoma longifolia Jack. J Fundam Appl Sci. 2017;9(2S):661-679.
- Thu H, Hussain Z, Mohamed I, Shuid A. Exploring dynamic biomedical algorithm of Eurycoma longifolia Jack and its bioactive phytochemicals: A review of pharmacokinetic and pharmacodynamic implications and future prospects. Asian Pac J Trop Med. 2018;11(2):89-97.
- Low B-S, Das PK, Chan K-L. Standardized quassinoid-rich Eurycoma longifolia extract improved spermatogenesis and fertility in male rats via the hypothalamic–pituitary–gonadal axis. J Ethnopharm. 2013;145(3):706-714.
- Thu HE, Hussain Z, Mohamed IN, Shuid AN. Eurycoma longifolia, a potential phytomedicine for the treatment of cancer: evidence of p53-mediated apoptosis in cancerous cells. Curr Drug Targets. 2018;19(10):1109-1126.
- Varghese CP, Ambrose C, Jin SC, Lim YJ, Keisaban T. Antioxidant and anti-inflammatory activity of Eurycoma longifolia Jack, a tracitional medicinal plant in Malaysia. Int J Pharm Sci Nanaotechnol. 2013;5(4):1875–1878.
- Tran TVA, Malainer C, Schwaiger S, et al. NF-B Inhibitors from Eurycoma longifolia. J Nat Prod. 2014;77(3):483-488.
- Li C-H, Liao J-W, Liao P-L, et al. Evaluation of acute 13-week subchronic toxicity and genotoxicity of the powdered root of tongkat ali (Eurycoma longifolia Jack). Evid Based Complement Alternat Med. 2013;2013:11.
- Talbott SM. Chapter 53 - Human Performance and Sports Applications of Tongkat Ali (Eurycoma longifolia). In: Bagchi D, Nair S, Sen CK, eds. Nutrition and Enhanced Sports Performance. San Diego: Academic Press; 2013:501-505.
- Ming YK, Zulkawi NB, Choudhary VK, Choudhary YK. Evaluation of the genotoxicity of Eurycoma longifolia aqueous extract (PHYSTA®) using in vitro ames test and in vivo mammalian micronucleus test. Int J Pharm Pharm Sci. 2014;6(4):652-657.
- Kiew OF, Singh R, Sirisinghe RG, Suen AB, Jamalullail SMS. Effects of a herbal drink on cycling endurance performance. Malays J Med Sci. 2003;10(1):78-85.
- Muhamad AS, Keong CC, Kiew OF, Abdullah MR, Lam CK. Effects of Eurycoma longifolia Jack supplementation on recreational athletes’ endurance running capacity and physiological responses in the heat. Int J Appl Sports Sci. 2010;22(2):1-19.
- Chen CK, Mohamad WMZW, Ooi FK, Ismail SB, Abdullah MR, George A. Supplementation of Eurycoma longifolia Jack extract for 6 weeks does not affect urinary testosterone: epitestosterone ratio, liver and renal functions in male recreational athletes. Int J Prev Med. 2014;5(6):728-733.
- George A, Udani J, Abidin NZ, Yusof A. Efficacy and safety of Eurycoma longifolia (Physta®) water extract plus multivitamins on quality of life, mood and stress: a randomized placebo-controlled and parallel study. Food Nutr Res. 2018;62:10.29219/fnr.v29262.21374.
- Hamzah S, Yusof A. The ergogenic effects of Eurycoma longifolia Jack: A pilot study. Br J Sports Med. 2003;37:464-470.
- Tambi MI. Standardized water soluble extract of Eurycoma longifolia (LJ100) on men’s health. In: Abstracts of the 8th International Congress of Andrology, 12–16 June 2005, Seoul, Korea. Int J Androl 2005;28(Suppl. 1):27.
- Talbott S, Talbott J, Negrete J, Jones M, Nichols M, Roza J. Effect of Eurycoma longifolia extract on anabolic balance during endurance exercise. J Int Soc Sports Nutr. 2006;3(1):S32.
- Sarina MY, Zaiton Z, Aminudin AHK, Nor AK, Azizol AK. Effects of resistance training and Eurycoma longifolia on muscle strength, lipid profile, blood glucose, and hormone level in middle-aged women. In: Abstracts from 4th Asia-Pacific Conference on Exercise and Sports Science & 8th International Sports Science Conference. Kuala Lumpur, Malaysia: Academy of Medicine of Malaysia; 2009.
- Tambi MIBM, Imran MK. Eurycoma longifolia Jack in managing idiopathic male infertility. Asian J Androl. 2010;12(3):376-380.
- Talbott S, Talbott J, Christopulos AM, Ekberg C, Larsen W, Jackson V. Ancient wisdom meets modern ailment - Traditional Asian medicine improves psychological Vigor in stressed subjects. Prog Nutr. 2010;12(1):64-69.
- Tambi MI, Imran MK, Henkel RR. Standardised water-soluble extract of Eurycoma longifolia, Tongkat ali, as testosterone booster for managing men with late-onset hypogonadism? Andrologia. 2012;44(Suppl 1):226–230.
- Ismail SB, Wan Mohammad WMZ, George A, Nik Hussain NH, Musthapa Kamal ZM, Liske E. Randomized clinical trial on the use of PHYSTA freeze-dried water extract of Eurycoma longifolia for the improvement of quality of life and sexual well-being in men. Evid Based Complement Alternat Med. 2012;2012:10.
- Henkel RR, Wang R, Bassett SH, et al. Tongkat ali as a potential herbal supplement for physically active male and female seniors—A pilot study. Phytother Res. 2014;28(4):544-550.
- Udani JK, George AA, Musthapa M, Pakdaman MN, Abas A. Effects of a proprietary freeze-dried water extract of Eurycoma longifolia (Physta) and Polygonum minus on sexual performance and well-being in men: A randomized, double-blind, placebo-controlled study. Evid Based Complement Alternat Med. 2014;2014:10.
- George A, Suzuki N, Abas AB, et al. Immunomodulation in middle-aged humans via the ingestion of Physta® standardized root water extract of Eurycoma longifolia Jack—A randomized, double-blind, placebo-controlled, parallel study. Phytother Res. 2016;30(4):627-635.
- Anon. Shitek Tongkat Ali Plus 400mg: Presence of tadalafil. WHO Pharmaceuticals Newsletter. 2004(3):2.
- Venhuis BJ, Tan J, Vredenbregt MJ, Ge X, Low M-Y, de Kaste D. Capsule shells adulterated with tadalafil. Forensic Sci Int. 2012;214(1):e20-e22.
- Do TTK, Theocharis G, Reich E. Simultaneous detection of three phosphodiesterase type 5 inhibitors and eight of their analogs in lifestyle products and screening for adulterants by high-performance thin-layer chromatography. J AOAC Int. 2015;98(5):1226-1233.
- Abubakar BM, Salleh FM, Shamsir Omar MS, Wagiran A. Assessing product adulteration of Eurycoma longifolia (Tongkat ali) herbal medicinal product using DNA barcoding and HPLC analysis. Pharm Biol. 2018;56(1):368-377.
- Glatter R. Viagra in your coffee? FDA recalls latest combo product. Forbes. July 21, 2017. Available at: www.forbes.com/sites/robertglatter/2017/07/21/viagra-in-your-coffee-fda-recalls-latest-combo-product/. Accessed April 29, 2019.
- Caverflo.com Issues Voluntary Nationwide Recall of Caverflo Natural Herbal Coffee due to the Presence of Undeclared Active Pharmaceutical Ingredients and Undeclared Milk [press release]. Silver Spring, MD: US Food and Drug Administration; December 1, 2018.
- Vejayan J, Mohamed AN, Zulkifli AA, Che Yahya YA, Munir N, Yusoff MM. Marker to authenticate Eurycoma longifolia (tongkat ali) containing aphrodisiac herbal products. Curr Sci. 2018;115(5):886-894.
- Ministry of Natural Resources and Environment. 4th National Report to the Convention on Biological Diversity. Putrajaya, Malaysia: Government of Malaysia; 2009.
- Sarawak Government. Laws of Sarawak. Wild Life Protection Ordinance 1998. Kuching, Sarawak: Percetakan Nasional Malaysia Berhad; 1998.
- Prime Minister’s Office Lao People’s Democratic Republic. Decree on natural resources for medicines (pharmaceutical natural resources) No 155/PM. Vientiane Capital Lao PDR: Prime Minister’s Office; 2003.
- Department of Environment and Natural Resources. Updated National List of Threatened Philippine Plants and Their Categories. Quezon City, the Philippines: Republic of the Philippines Department of Environment and Natural Resources; 2017.
- Lee HS. Introducing the cultivation of medicinal plants and wild fruits in forest rehabilitation operations on former shifting cultivation sites in Sarawak Malaysia: Issues and challenges. Southeast Asian Stud. 2004;42(1):60-73.
- Ali ARM, Norini H, Lim HF. The role of forestry research and development (R&D) institution in policy formulation and implementation: A Malaysian perspective. Pertanika J Trop Agric Sci. 2007;30(2):153-163.
- Anon. Luasong tree replanting project a success. Borneo Post Online. December 5, 2018.
- Hussein S, Ling APK, Ng TH, Ibrahim R, Paek KY. Adventitious roots induction of recalcitrant tropical woody plant, Eurycoma longifolia. Romanian Biotechnol Lett. 2012;17(1):7026-7035.
- Rahim KA, Ibrahim R, Othman Z, Harun AR, eds. A Compendium of R&D on Nuclear Technology Applications in Agriculture and Biosciences (1984-2014). Selangor, Malaysia: Malaysian Nuclear Agency; 2014.
- Hung TN, Chi VL. Country Status Report on Medicinal and Aromatic Plants in Vietnam. In: Paroda R, Dasgupta S, Mal B, Ghosh SP, Pareek SK, eds. Expert Consultation on Promotion of Medicinal and Aromatic Plants in the Asia-Pacific Region: Proceedings, Bangkok, Thailand; 2-3 December, 2013. Bangkok, Thailand: Asia-Pacific Association of Agricultural Research Institutions (APAARI); 2014:226-231.
- Idris MAH, Haron N. Supply and demand of Eurycoma longifolia (tongkat ali) plants with few others. J Trop Med Plants. 2001;2(1):145-154.
- Vijaindren A. Flaccid outlook for tongkat ali. New Sunday Times. 2009:16.
- Jabatan Perkhidmatan Kuarantin dan Pemeriksaan Malaysia (MAQIS). Prosedur Operasi Piawaian: Pemeriksaan Konsainan Herba yang Dieksport [Standard Operating Procedure: Consignment Inspection for Exported Herbs] [in Malay]. Putrajaya, Malaysia: MAQIS; 2014.
- Wan-Muhammad-Azrul WA, Mohd-Farid A, Lee SY, Sajap AS, Omar D, Mohamed R. A survey on the occurrence of pests and diseases in tongkat ali (Eurycoma longifolia) plantations in Peninsular Malaysia. J Trop For Sci. 2018;30(3):362-375.
|