|
|
|
|
|
|
|
|
|
Issue: 30 Page: 33
ECHINACEA: A LITERATURE REVIEW; BOTANY, HISTORY, CHEMISTRY, PHARMACOLOGY, TOXICOLOGY, AND CLINICAL USES.
by Christopher Hobbs
HerbalGram. 1994; 30:33 American Botanical Council
HINACEA: A LITERATURE REVIEW; BOTANY, HISTORY, CHEMISTRY, PHARMACOLOGY, TOXICOLOGY, AND CLINICAL USES ABSTRACT: Medicinal use of Echinacea species has been extensive and is growing. This article reviews the literature on these plants concerning botany, history of use, chemistry, physiological effects, clinical applications, pharmacognosy, cultivation, and safety.
INTRODUCTION
Knowledge about many of the popular medicinal plants from North America in common use today derives from the Native Americans. Many tribes had thousands of years of direct experience with herbs. In their culture, and in later early American-European culture, several of these herbs are of special interest -- but especially herbs from the genus indigenous only to North America, Echinacea.
Samples of Echinacea have been found in archeological digs of Lakota Sioux village sites from the 1600s (Wedel, 1936). Echinacea has experienced cycles of increased popularity in the time it has been known to the European-based settlers, from the turn of the 19th century.
Currently there is a reawakened interest in echinacea in the United States. The last few years have seen a tremendous rise in popularity from relative obscurity between 1930 and 1980 partly due to increased interest in immune system functions. Echinacea is probably one of the most promising immune strengtheners and modulators, with numerous scientific studies and rich clinical evidence in its favor. Over the last 99 years, various echinacea species have had over 400 journal articles to their credit.
Twenty years after its "discovery" by the lay doctor H. C. F. Meyer and introduction into the Materia Medica by the Eclectic doctor John King and John Uri Lloyd (ca. 1887), it had become the number one herb in popularity among both Eclectic and Regular medical doctors. Although orthodox medicine officially refused to recognize its worth and it was surrounded by controversy, many physicians used it, defending its efficacy. The centennial of its "discovery" by the medical profession in the U.S. comes at a time when its popularity has never been greater.
BOTANY
ORIGIN OF THE NAMES AND TAXONOMY
Echinacea is one of the coneflowers, a group of native American wildflowers from the Daisy Family (Asteraceae) characterized by spiny flowering heads, with an elevated receptacle which forms the "cone." Other genera in this group, the Heliantheae, the largest tribe in the Asteraceae, include Rudbeckia L., Ratibida Raf., and Dracopis Cass. (Stuessy, 1977). However, newer work has resulted in the placement of Echinacea in another tribe, the Ecliptinae, while the other three genera were placed into a new subtribe, Rudbeckiinae (Robinson, 1981).
Echinacea has only a few common names in English. The most widely encountered common name is Purple Coneflower, for obvious reasons. One also sees Purple Kansas Coneflower, Black Sampson, Red Sunflower, Comb Flower, Cock Up Hat, Missouri Snakeroot and Indian Head Lyons 1907). Echinacea purpurea(L.) Moench has been popular in American horticulture as a border plant or as a plant in wild gardens for many years. A number of cultivars have been selected, varying in flower size and color, among other characteristics. Some of the more popular include Brightling, Golden, Queen, Leuchstern, Masterpiece, Rosequeen, Scarletta, The King (Bright crimson rays of good substance), The Pilot, Winchmore Hill, and Sombrero (bushier and with larger crimson-purple rays) (Kelsey, 1942; Dress, 1961).
A number of Latin names have been used for the plants now accepted as being from the genus Echinacea. The Purple Coneflower was known to European botanists as early as the 1690s and may have been first collected in Virginia (and sent to European botanists) by the Reverend John Banister in 1680-2 (Morison, 1699). In his Plantarum Historiae Universalis Oxoniensis (part 3), Robert Morison, the first Professor of Botany at Oxford University, calls the Purple Coneflower "Bite of the Devil" and gives it the first "official" name: Dracunculus virginianus latifolius, petalis florum longissimis purpurascentibus, which roughly translated means, "The Little Dragon of Virginia, having flowers with long, reddish-purple petals projecting out to the side."
Other botanists (e.g., Herman, Catesby, Dillenius, and Clayton) described the plant in the early 18th century but it was Linnaeus (1753), the great Swedish botanist and physician, who named the Purple Coneflower, Rudbeckia purpurea (=Echinacea purpurea) after Olaf Rudbeck, a fellow botanist and physician. This name was used in the botanical and horticultural literature as late as 1860, and is even occasionally found in contemporary literature.
Today, the purple coneflowers are placed in the genus Echinacea, first described by Moench (1794). This name replaced Brauneria previously used by de Necker in 1790 (Britton, 1896), but later discredited. Table I summarizes the origin and first descriptions of all nine currently accepted Echinacea species.
The most complete recent taxonomic treatment of the genus Echinacea is the work of Ronald L. McGregor from the University of Kansas (1968). He describes several varieties and includes data on chromosome numbers, comparative anatomy, and hybridization among Echinacea species. Hybridization and chromosome work may be important in the future in the development of more effective strains for medicinal use. The chromosome number can affect the amount of secondary compounds that a plant produces, and populations with preferred chromosome numbers may be found in the wild, or developed, to be used in the manufacture of botanical medicines.
Because E. pallida is still confused with E. angustifolia in commercial trade, exact chemical and morphological criteria for distinguishing samples in commerce have been worked out by Bauer, Heubl, and others (Heubl et al., 1988; Heubl & Bauer, 1990).
DESCRIPTION
Plants from the genus Echinacea are herbaceous perennials whose stems are simple or branched, upright, and ascend from either a vertical taproot (Echinacea angustifolia) or branched, more fibrous roots (E. purpurea). The plants are either glaucous (waxy) and smooth, or variously hairy, usually with coarse hairs. The leaves are petiolate (stalked) below, becoming sessile (stalkless) and smaller above, and are prominently 3-5 veined, either ovate, ovate lanceolate, elliptical and coarsely toothed or entire.
The flowering heads are single at the end of the stems or branches, having both ray and disc flowers, of which a prominent characteristic is the conical or hemispheric "cone" of the receptacle. The phyllaries (subtending bracts of the head) are in 3-4 series, imbricated (overlapping), the outer ones more leaf-like, lance-shaped to lance-linear, transitioning into the receptacle spines (called pales). These exceed the flowers in length (a key character and genus trait) and end in sharp or blunt spines. The ray flowers are sterile, in one series, strap-shaped, 2- or 3-notched at the end, sometimes reflexed characteristically downward, usually rose-colored or purple, sometimes white or yellow (Echinacea paradoxa (Norton) Britton) to red. The disc flowers are fertile. the corolla expanding below into a fleshy bulb-like base. while the tube is cylindrical and has a 5-lobed erect limb. The achenes (seeds) are 4-angled, 3-3.5 mm long, and the pappus consists of a short, smooth or tooth ed crown. McGregor's key to the species can be found in his original paper (1968) and is reprinted in The Echinacea Handbook (Hobbs, 1989). See this work and Foster (1991) for more complete information on the botany and taxonomy of the genus.
ABSTRACT: Medicinal use of Echinacea species has been extensive and is growing. This article reviews the literature on these plants concerning botany, history of use, chemistry, physiological effects, clinical applications, pharmacognosy, cultivation, and safety.
INTRODUCTION
Knowledge about many of the popular medicinal plants from North America in common use today derives from the Native Americans. Many tribes had thousands of years of direct experience with herbs. In their culture, and in later early American-European culture, several of these herbs are of special interest -- but especially herbs from the genus indigenous only to North America, Echinacea.
Samples of Echinacea have been found in archeological digs of Lakota Sioux village sites from the 1600s (Wedel, 1936). Echinacea has experienced cycles of increased popularity in the time it has been known to the European-based settlers, from the turn of the 19th century.
Currently there is a reawakened interest in echinacea in the United States. The last few years have seen a tremendous rise in popularity from relative obscurity between 1930 and 1980 partly due to increased interest in immune system functions. Echinacea is probably one of the most promising immune strengtheners and modulators, with numerous scientific studies and rich clinical evidence in its favor. Over the last 99 years, various echinacea species have had over 400 journal articles to their credit.
Twenty years after its "discovery" by the lay doctor H. C. F. Meyer and introduction into the Materia Medica by the Eclectic doctor John King and John Uri Lloyd (ca. 1887), it had become the number one herb in popularity among both Eclectic and Regular medical doctors. Although orthodox medicine officially refused to recognize its worth and it was surrounded by controversy, many physicians used it, defending its efficacy. The centennial of its "discovery" by the medical profession in the U.S. comes at a time when its popularity has never been greater.
BOTANY
ORIGIN OF THE NAMES AND TAXONOMY
Echinacea is one of the coneflowers, a group of native American wildflowers from the Daisy Family (Asteraceae) characterized by spiny flowering heads, with an elevated receptacle which forms the "cone." Other genera in this group, the Heliantheae, the largest tribe in the Asteraceae, include Rudbeckia L., Ratibida Raf., and Dracopis Cass. (Stuessy, 1977). However, newer work has resulted in the placement of Echinacea in another tribe, the Ecliptinae, while the other three genera were placed into a new subtribe, Rudbeckiinae (Robinson, 1981).
Echinacea has only a few common names in English. The most widely encountered common name is Purple Coneflower, for obvious reasons. One also sees Purple Kansas Coneflower, Black Sampson, Red Sunflower, Comb Flower, Cock Up Hat, Missouri Snakeroot and Indian Head Lyons 1907). Echinacea purpurea(L.) Moench has been popular in American horticulture as a border plant or as a plant in wild gardens for many years. A number of cultivars have been selected, varying in flower size and color, among other characteristics. Some of the more popular include Brightling, Golden, Queen, Leuchstern, Masterpiece, Rosequeen, Scarletta, The King (Bright crimson rays of good substance), The Pilot, Winchmore Hill, and Sombrero (bushier and with larger crimson-purple rays) (Kelsey, 1942; Dress, 1961).
A number of Latin names have been used for the plants now accepted as being from the genus Echinacea. The Purple Coneflower was known to European botanists as early as the 1690s and may have been first collected in Virginia (and sent to European botanists) by the Reverend John Banister in 1680-2 (Morison, 1699). In his Plantarum Historiae Universalis Oxoniensis (part 3), Robert Morison, the first Professor of Botany at Oxford University, calls the Purple Coneflower "Bite of the Devil" and gives it the first "official" name: Dracunculus virginianus latifolius, petalis florum longissimis purpurascentibus, which roughly translated means, "The Little Dragon of Virginia, having flowers with long, reddish-purple petals projecting out to the side."
Other botanists (e.g., Herman, Catesby, Dillenius, and Clayton) described the plant in the early 18th century but it was Linnaeus (1753), the great Swedish botanist and physician, who named the Purple Coneflower, Rudbeckia purpurea (=Echinacea purpurea) after Olaf Rudbeck, a fellow botanist and physician. This name was used in the botanical and horticultural literature as late as 1860, and is even occasionally found in contemporary literature.
Today, the purple coneflowers are placed in the genus Echinacea, first described by Moench (1794). This name replaced Brauneria previously used by de Necker in 1790 (Britton, 1896), but later discredited. Table I summarizes the origin and first descriptions of all nine currently accepted Echinacea species.
The most complete recent taxonomic treatment of the genus Echinacea is the work of Ronald L. McGregor from the University of Kansas (1968). He describes several varieties and includes data on chromosome numbers, comparative anatomy, and hybridization among Echinacea species. Hybridization and chromosome work may be important in the future in the development of more effective strains for medicinal use. The chromosome number can affect the amount of secondary compounds that a plant produces, and populations with preferred chromosome numbers may be found in the wild, or developed, to be used in the manufacture of botanical medicines.
Because E. pallida is still confused with E. angustifolia in commercial trade, exact chemical and morphological criteria for distinguishing samples in commerce have been worked out by Bauer, Heubl, and others (Heubl et al., 1988; Heubl & Bauer, 1990).
DESCRIPTION
Plants from the genus Echinacea are herbaceous perennials whose stems are simple or branched, upright, and ascend from either a vertical taproot (Echinacea angustifolia) or branched, more fibrous roots (E. purpurea). The plants are either glaucous (waxy) and smooth, or variously hairy, usually with coarse hairs. The leaves are petiolate (stalked) below, becoming sessile (stalkless) and smaller above, and are prominently 3-5 veined, either ovate, ovate lanceolate, elliptical and coarsely toothed or entire.
The flowering heads are single at the end of the stems or branches, having both ray and disc flowers, of which a prominent characteristic is the conical or hemispheric "cone" of the receptacle. The phyllaries (subtending bracts of the head) are in 3-4 series, imbricated (overlapping), the outer ones more leaf-like, lance-shaped to lance-linear, transitioning into the receptacle spines (called pales). These exceed the flowers in length (a key character and genus trait) and end in sharp or blunt spines. The ray flowers are sterile, in one series, strap-shaped, 2- or 3-notched at the end, sometimes reflexed characteristically downward, usually rose-colored or purple, sometimes white or yellow (Echinacea paradoxa (Norton) Britton) to red. The disc flowers are fertile. the corolla expanding below into a fleshy bulb-like base. while the tube is cylindrical and has a 5-lobed erect limb. The achenes (seeds) are 4-angled, 3-3.5 mm long, and the pappus consists of a short, smooth or tooth ed crown. McGregor's key to the species can be found in his original paper (1968) and is reprinted in The Echinacea Handbook (Hobbs, 1989). See this work and Foster (1991) for more complete information on the botany and taxonomy of the genus. HISTORY OF USE
ETHNOBOTANY
Many Native American tribes had a substantial pharmacopoeia, and some used herbs and other internal medicines extensively. In the early days of European colonization of the North American continent, native peoples were known to share their considerable skill with the Europeans (Vogel, 1970), who generally were in need of medicines, due to the difficulty of transporting official drugs across the Atlantic. Of all the indigenous medicines introduced by Native Americans, echinacea may be one of the most important.
According to Gilmore (1911, 1913), "Echinacea seems to have been used as a remedy for more ailments than any other plant." Meyer, the German lay physician who first introduced echinacea to the medical profession, learned of its healing virtues from Native Americans (possibly the Pawnee or Omaha) living in Nebraska at the time. Table 2 lists all the tribal uses reported in the literature. For a thorough review of the ethnobotany of echinacea, see Hobbs (1989) and Foster (1991).
As can be seen from the map (Fig. 1), most of the information we have on the ethnobotany of Echinacea comes from tribes that roamed the great plains region.
EARLY EUROPEAN-AMERICAN AND ECLECTIC USES
Little mention was made of echinacea in medical literature before H. F. C. Meyer, a lay doctor from Nebraska, "discovered" the virtues of E. angustifolia around the 1870s and began making a patent medicine from it.
The earliest reference to the medicinal uses of any of the species of Echinacea appeared in the second edition of Flora Virginica by L. T. Gronovius (1762), derived from the notes of John Clayton (1693-1773), an English botanist who lived in Virginia for 40 years. Clayton states that E. purpurea "bears a sharp-tasting root and is very valuable in treating the saddle-sores of horses" (Berkeley & Berkeley 1963; Foster 1991). Rafinesque (1830) mentions that the Sioux used Echinacea for syphilis, and Riddell (1835) notes of E. purpurea, "root thick, black, very pungent to the taste; aromatic and carminative, little known." Comings (1847), in perhaps the first journal article on echinacea, says that "the tincture or decoction is a specific for the venereal disease in its worst forms, having never been perserveringly employed without success." The noted American botanist, Asa Gray, wrote in his Manual of Botany (1848) that E. purpurea has a "root thick, black, very pungent to the tas te, used in popular medicine under the name of black sampson." He later said that it was "called black sampson by quack doctors" (Gray, 1879).
HISTORY OF USE
ETHNOBOTANY
Many Native American tribes had a substantial pharmacopoeia, and some used herbs and other internal medicines extensively. In the early days of European colonization of the North American continent, native peoples were known to share their considerable skill with the Europeans (Vogel, 1970), who generally were in need of medicines, due to the difficulty of transporting official drugs across the Atlantic. Of all the indigenous medicines introduced by Native Americans, echinacea may be one of the most important.
According to Gilmore (1911, 1913), "Echinacea seems to have been used as a remedy for more ailments than any other plant." Meyer, the German lay physician who first introduced echinacea to the medical profession, learned of its healing virtues from Native Americans (possibly the Pawnee or Omaha) living in Nebraska at the time. Table 2 lists all the tribal uses reported in the literature. For a thorough review of the ethnobotany of echinacea, see Hobbs (1989) and Foster (1991).
As can be seen from the map (Fig. 1), most of the information we have on the ethnobotany of Echinacea comes from tribes that roamed the great plains region.
EARLY EUROPEAN-AMERICAN AND ECLECTIC USES
Little mention was made of echinacea in medical literature before H. F. C. Meyer, a lay doctor from Nebraska, "discovered" the virtues of E. angustifolia around the 1870s and began making a patent medicine from it.
The earliest reference to the medicinal uses of any of the species of Echinacea appeared in the second edition of Flora Virginica by L. T. Gronovius (1762), derived from the notes of John Clayton (1693-1773), an English botanist who lived in Virginia for 40 years. Clayton states that E. purpurea "bears a sharp-tasting root and is very valuable in treating the saddle-sores of horses" (Berkeley & Berkeley 1963; Foster 1991). Rafinesque (1830) mentions that the Sioux used Echinacea for syphilis, and Riddell (1835) notes of E. purpurea, "root thick, black, very pungent to the taste; aromatic and carminative, little known." Comings (1847), in perhaps the first journal article on echinacea, says that "the tincture or decoction is a specific for the venereal disease in its worst forms, having never been perserveringly employed without success." The noted American botanist, Asa Gray, wrote in his Manual of Botany (1848) that E. purpurea has a "root thick, black, very pungent to the tas te, used in popular medicine under the name of black sampson." He later said that it was "called black sampson by quack doctors" (Gray, 1879).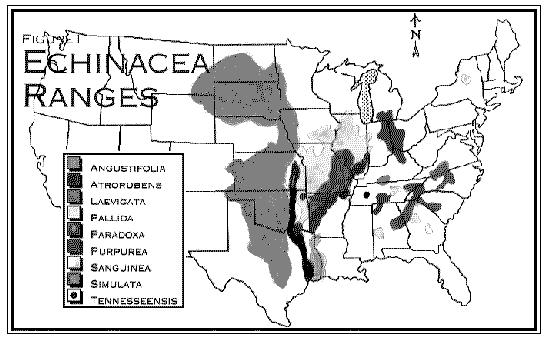 THE ECLECTICS
The Eclectics, prominent in the United States from 1845 to the 1930s, were a group of medical doctors who employed botanical medicine extensively in their practices. In their heyday they maintained a number of medical schools in various parts of the United States -- most notably in Cincinnati -- and claimed practitioners in every part of the country.
The Eclectic school was a major force in bringing Echinacea to the forefront of herbal medicine. John King, one of the best-known Eclectic doctors and author of the important American Dispensatory (1852), together with John Uri Lloyd, an eminent pharmacist, writer, and manufacturer, was instrumental in introducing Echinacea to the medical profession in 1887. The history of the introduction of E. angustifolia into mainstream medicine has been quoted widely; the best accounts are Lloyd's own History of Echinacea angustifolia (1904), and the Lloyd brother's A Treatise on Echinacea (1917).
At first King and Lloyd were incredulous, because Meyer, the German lay doctor who sent the plant to them, made such exaggerated claims of its miraculous healing powers, most of which were too wild to be believed. They ended up taking back their words after reluctantly trying it, and echinacea eventually became the most popular herb of the entire Eclectic era.
The Eclectics used Lloyd's famous Echafolta, a clear, high-alcohol preparation, as well as his "Specific Medicine Echinacea," in addition to commercial products from many other drug companies, such as Merck, Wyeth and Parke, Davis. These preparations were recommended for a wide range of ailments, many of which involved anti-microbial and anti-toxin effects. Specifically, its virtues were extolled for insect bites, snake and spider bites, the bites of rabid dogs, diphtheria, typhoid, carbuncles, cerebro-spinal meningitis complicated by herpes, blood-poisoning, puerperal septicemia, gonorrhea, eczema, syphilis, and pyemia (King & Meyer, 1887; Hobbs, 1989). Unruh (1915) even foreshadowed our current knowledge of the action of echinacea as an immune stimulant, using it in a manner to treat tuberculosis, reporting that it increased the phagocytic powers of the leukocytes, similar to vaccines.
THE REGULARS
The "regular" medical establishment at the time openly criticized the use of echinacea (Chamberlin, 1905; Couch & Giltner, 1921a, b), and the Journal of the American Medical Association ran articles that declared it a useless "quack remedy." Meanwhile, as a number of official organizations were criticizing it, many physicians were using it in clinical practice and extolling its virtues (Thackeray, 1923; Editorial, 1930), and ironically, it was included in the 1916 United States National Formulary, an inclusion which lasted until 1950. Reports by doctors in the medical literature of the time show that successes were seen with puerperal fever "where all else fails," teeth and gum disease, boils, typhoid, anthrax, and even in veterinary practice (Webster, 1891). For a comprehensive review of the medical articles see Hobbs (1989) and Foster (1991).
CHEMISTRY
HISTORY Of CHEMICAL ANALYSIS
It is fitting that the first published report on the chemical constituents of Echinacea was by John Uri Lloyd, in 1897. Before that time, 11 years after its introduction into general medical practice, little was known of its chemical makeup. References were made only that it was at first "sweet," and then "acrid." This in itself says something about its chemistry, for we could surmise that the root might contain sugars and perhaps essential oil, which it does. The methods of assay were crude, however, as was evidenced by Couch and Giltner's study (1921a, 1921b). They had no idea at that time the depth of Echinacea's chemical profile and physiological activity.
Despite the crude methods available to him, Lloyd was thorough in his investigation of E. angustifolia and submitted it to extensive tests. After completing his analysis, he reported that "echinacea contains minute traces of a colorless alkaloid...." Although it is likely that other compounds, besides alkaloids, may have been responsible for Lloyd's positive alkaloid test (Bauer, 1992), a German research team (Röder, et al.), detected traces (.0065 in dried roots) of the pyrrolizidine alkaloids tussilagin and isotussilagin in E. purpurea and E. angustifolia.
Today a great deal more is known about the chemical makeup of various Echinacea species, thanks to a number of groups working in Germany, particularly to Professor H. Wagner and his students at the Institute of Pharmaceutical Biology in Munich. One of the researchers, Rudi Bauer, has done a tremendous amount of work to legitimize the popular and medical uses of the genus. Although there is undoubtedly more to learn about the active constituents of echinacea, we do know that among the many compounds that have been already identified, the alkylamides, cichoric acid derivatives, polysaccharides and possibly glycoproteins are the most promising. Other major groups of compounds that have been found in various echinacea species include flavonoids, monoterpenes, a number of caffeic acid derivatives, hydrocarbons such as N-alkanes, polyacetylenes, high molecular-weight polysaccharides, and traces of pyrrolizidine alkaloids. A complete summary of the chemistry and pharmacology of the spe cies that have been studied to date can be reviewed in a separate publication available from ABC, P. O. Box 201660, Austin, TX 78720, for $5.00, postpaid.
For a more complete review of the past and present investigations on the chemistry of Echinacea species, see Bauer and Wagner (1990, 1991) or Hobbs (1989).
Note: Analytical work (circa 1955 to 1987) on E. angustifolia must be called into question, because of possible misidentification of the species. It is possible that E. angustifolia could have actually been E. pallida (which, however, was also official in the National Formulary (1916-1946)), or even other species that were not clearly differentiated by early researchers. Early work on E. angustifolia in Germany could have also been E. pallida, and any work on commercial E. purpurea roots (not E. purpurea tops or work on the product Echinacin(R), where the plants were grown by the manufacturer) could have actually been performed on Parthenium integrifolium, a common adulterant after about 1910 (along with Eryngium aquaticum and Lespedeza capitata). Work that falls into this category is marked as such in the table (Moser, 1910; Lloyd, 1917).
PHARMACOLOGY
Eclectic doctors learned of the potential of echinacea from the Native Americans (through Meyer) and developed the clinical use of it in this country. Homeopaths brought it to Europe (Wood, 1925), and began experimenting with it and writing about it in the 1920s and 1930s. Gerhard Madaus, founder of the pharmaceutical manufacturing firm, Madaus AG, was the first to report on his clinical and laboratory work with the genus. Auster & Schafer (1957) report that Madaus went to the U.S. for seeds of E. angustifolia, which was the best-known and studied species of the time, only to discover later that he was growing E. purpurea. This was quite fortuitous, because the former plant will not grow nearly as well in Germany as the latter -- and some studies suggest that E. purpurea has shown stronger immune-stimulating properties than E. angustifolia. They are probably comparable in strength.
From this beginning and the first work in the laboratory in Germany in the 1930s, there have been over 400 journal articles published on the chemistry, pharmacology and clinical uses of echinacea to date.
Because of space limitations in this review, the major pharmacological activities will be summarized in a separate publication. See page 48 for details.
For more complete reviews see Schimmel and Werner, 1981 (Echinacin(R)); Bauer and Wagner, 1990, 1991; Hobbs, 1989; Foster, 1991.
MODERN CLINICAL WORK
With all the interest in echinacea and its commercial preparations, it is interesting that there have been only a few controlled double-blind clinical trials to verify the cultural and medical uses.
However, there have been numerous clinical reports about the use of echinacea, nearly all with the fresh stabilized E. purpurea juice, Echinacin(R), in the injectible form, although a few have been conducted with oral applications or an externally applied salve. As interesting and suggestive as these studies are, they are not indicative of clinical application outside of Europe (especially the U.S., Canada, New Zealand, and Australia where oral echinacea preparations are popular), where echinacea in this form is rarely, if eve,r used. A few other studies have been performed with combination products such as Esberitox(R), which, besides echinacea, contains extracts of Baptisia tinctoria (Wild indigo), and Thuja occidentalis (Arbor vitae).There are over 200 studies to report here, so only representative citations will be given in Table 2. In nearly every case, a favorable outcome was reported with the echinacea treatment. Many of these trials are uncontrolled and would not stand u p to today's strict standards for clinical testing; however, they do have merit in representing actual clinical experience with the preparation. For a comprehensive review, see Hobbs (1989) and Foster (1991).
THE ECLECTICS
The Eclectics, prominent in the United States from 1845 to the 1930s, were a group of medical doctors who employed botanical medicine extensively in their practices. In their heyday they maintained a number of medical schools in various parts of the United States -- most notably in Cincinnati -- and claimed practitioners in every part of the country.
The Eclectic school was a major force in bringing Echinacea to the forefront of herbal medicine. John King, one of the best-known Eclectic doctors and author of the important American Dispensatory (1852), together with John Uri Lloyd, an eminent pharmacist, writer, and manufacturer, was instrumental in introducing Echinacea to the medical profession in 1887. The history of the introduction of E. angustifolia into mainstream medicine has been quoted widely; the best accounts are Lloyd's own History of Echinacea angustifolia (1904), and the Lloyd brother's A Treatise on Echinacea (1917).
At first King and Lloyd were incredulous, because Meyer, the German lay doctor who sent the plant to them, made such exaggerated claims of its miraculous healing powers, most of which were too wild to be believed. They ended up taking back their words after reluctantly trying it, and echinacea eventually became the most popular herb of the entire Eclectic era.
The Eclectics used Lloyd's famous Echafolta, a clear, high-alcohol preparation, as well as his "Specific Medicine Echinacea," in addition to commercial products from many other drug companies, such as Merck, Wyeth and Parke, Davis. These preparations were recommended for a wide range of ailments, many of which involved anti-microbial and anti-toxin effects. Specifically, its virtues were extolled for insect bites, snake and spider bites, the bites of rabid dogs, diphtheria, typhoid, carbuncles, cerebro-spinal meningitis complicated by herpes, blood-poisoning, puerperal septicemia, gonorrhea, eczema, syphilis, and pyemia (King & Meyer, 1887; Hobbs, 1989). Unruh (1915) even foreshadowed our current knowledge of the action of echinacea as an immune stimulant, using it in a manner to treat tuberculosis, reporting that it increased the phagocytic powers of the leukocytes, similar to vaccines.
THE REGULARS
The "regular" medical establishment at the time openly criticized the use of echinacea (Chamberlin, 1905; Couch & Giltner, 1921a, b), and the Journal of the American Medical Association ran articles that declared it a useless "quack remedy." Meanwhile, as a number of official organizations were criticizing it, many physicians were using it in clinical practice and extolling its virtues (Thackeray, 1923; Editorial, 1930), and ironically, it was included in the 1916 United States National Formulary, an inclusion which lasted until 1950. Reports by doctors in the medical literature of the time show that successes were seen with puerperal fever "where all else fails," teeth and gum disease, boils, typhoid, anthrax, and even in veterinary practice (Webster, 1891). For a comprehensive review of the medical articles see Hobbs (1989) and Foster (1991).
CHEMISTRY
HISTORY Of CHEMICAL ANALYSIS
It is fitting that the first published report on the chemical constituents of Echinacea was by John Uri Lloyd, in 1897. Before that time, 11 years after its introduction into general medical practice, little was known of its chemical makeup. References were made only that it was at first "sweet," and then "acrid." This in itself says something about its chemistry, for we could surmise that the root might contain sugars and perhaps essential oil, which it does. The methods of assay were crude, however, as was evidenced by Couch and Giltner's study (1921a, 1921b). They had no idea at that time the depth of Echinacea's chemical profile and physiological activity.
Despite the crude methods available to him, Lloyd was thorough in his investigation of E. angustifolia and submitted it to extensive tests. After completing his analysis, he reported that "echinacea contains minute traces of a colorless alkaloid...." Although it is likely that other compounds, besides alkaloids, may have been responsible for Lloyd's positive alkaloid test (Bauer, 1992), a German research team (Röder, et al.), detected traces (.0065 in dried roots) of the pyrrolizidine alkaloids tussilagin and isotussilagin in E. purpurea and E. angustifolia.
Today a great deal more is known about the chemical makeup of various Echinacea species, thanks to a number of groups working in Germany, particularly to Professor H. Wagner and his students at the Institute of Pharmaceutical Biology in Munich. One of the researchers, Rudi Bauer, has done a tremendous amount of work to legitimize the popular and medical uses of the genus. Although there is undoubtedly more to learn about the active constituents of echinacea, we do know that among the many compounds that have been already identified, the alkylamides, cichoric acid derivatives, polysaccharides and possibly glycoproteins are the most promising. Other major groups of compounds that have been found in various echinacea species include flavonoids, monoterpenes, a number of caffeic acid derivatives, hydrocarbons such as N-alkanes, polyacetylenes, high molecular-weight polysaccharides, and traces of pyrrolizidine alkaloids. A complete summary of the chemistry and pharmacology of the spe cies that have been studied to date can be reviewed in a separate publication available from ABC, P. O. Box 201660, Austin, TX 78720, for $5.00, postpaid.
For a more complete review of the past and present investigations on the chemistry of Echinacea species, see Bauer and Wagner (1990, 1991) or Hobbs (1989).
Note: Analytical work (circa 1955 to 1987) on E. angustifolia must be called into question, because of possible misidentification of the species. It is possible that E. angustifolia could have actually been E. pallida (which, however, was also official in the National Formulary (1916-1946)), or even other species that were not clearly differentiated by early researchers. Early work on E. angustifolia in Germany could have also been E. pallida, and any work on commercial E. purpurea roots (not E. purpurea tops or work on the product Echinacin(R), where the plants were grown by the manufacturer) could have actually been performed on Parthenium integrifolium, a common adulterant after about 1910 (along with Eryngium aquaticum and Lespedeza capitata). Work that falls into this category is marked as such in the table (Moser, 1910; Lloyd, 1917).
PHARMACOLOGY
Eclectic doctors learned of the potential of echinacea from the Native Americans (through Meyer) and developed the clinical use of it in this country. Homeopaths brought it to Europe (Wood, 1925), and began experimenting with it and writing about it in the 1920s and 1930s. Gerhard Madaus, founder of the pharmaceutical manufacturing firm, Madaus AG, was the first to report on his clinical and laboratory work with the genus. Auster & Schafer (1957) report that Madaus went to the U.S. for seeds of E. angustifolia, which was the best-known and studied species of the time, only to discover later that he was growing E. purpurea. This was quite fortuitous, because the former plant will not grow nearly as well in Germany as the latter -- and some studies suggest that E. purpurea has shown stronger immune-stimulating properties than E. angustifolia. They are probably comparable in strength.
From this beginning and the first work in the laboratory in Germany in the 1930s, there have been over 400 journal articles published on the chemistry, pharmacology and clinical uses of echinacea to date.
Because of space limitations in this review, the major pharmacological activities will be summarized in a separate publication. See page 48 for details.
For more complete reviews see Schimmel and Werner, 1981 (Echinacin(R)); Bauer and Wagner, 1990, 1991; Hobbs, 1989; Foster, 1991.
MODERN CLINICAL WORK
With all the interest in echinacea and its commercial preparations, it is interesting that there have been only a few controlled double-blind clinical trials to verify the cultural and medical uses.
However, there have been numerous clinical reports about the use of echinacea, nearly all with the fresh stabilized E. purpurea juice, Echinacin(R), in the injectible form, although a few have been conducted with oral applications or an externally applied salve. As interesting and suggestive as these studies are, they are not indicative of clinical application outside of Europe (especially the U.S., Canada, New Zealand, and Australia where oral echinacea preparations are popular), where echinacea in this form is rarely, if eve,r used. A few other studies have been performed with combination products such as Esberitox(R), which, besides echinacea, contains extracts of Baptisia tinctoria (Wild indigo), and Thuja occidentalis (Arbor vitae).There are over 200 studies to report here, so only representative citations will be given in Table 2. In nearly every case, a favorable outcome was reported with the echinacea treatment. Many of these trials are uncontrolled and would not stand u p to today's strict standards for clinical testing; however, they do have merit in representing actual clinical experience with the preparation. For a comprehensive review, see Hobbs (1989) and Foster (1991).
 TOXICOLOGY
Historical human use and experimental animal studies indicate little need for concern regarding the safety of echinacea. Some toxicological work on the toxicity of echinacea has been done, mainly on the fresh tops of Echinacea purpurea For a review of toxicological studies, see the additional information in Chemistry/Pharmacy.
PHARMACY AND PHARMACOGNOSY
COMMERCIAL PREPARATIONS
As immunomodulators, the popularity of echinacea preparations is increasing worldwide. For instance, in Germany (1989), the most popular echinacea product is 131st on a list of the 2,000 most-prescribed drugs (Schumacher & Friedberg, 1991), and is possibly the most popular herb in the U.S, with sales increasing dramatically over the last few years. It is obvious to the herbal consumer that there is an increasing array of echinacea products available in the natural food store or herb shop. The use of echinacea seems to "cross over" into groups of people who wouldn't normally use herbal products, and even Hollywood has discovered echinacea. It was recently reported in the popular press that celebreties such as Cher, Jodie Foster, and members of the Star Trek cast commonly use the herb to ward off colds and flus (Garstang, 1993). Much of this popularity seems to be due to simple word-of-mouth transmission.
At present, the most popular echinacea products are liquid extracts (made with varying proportions of grain alcohol and water, and, in some cases, glycerin), spray- or freeze-dried extracts in capsules and tablets, simple herb powders in capsules and tablets and the fresh juice of E. purpurea tops stabilized with ethanol.
Of the proven immuno-active compounds from Echinacea spp., the polyacetylenes and cichoric acid are unstable and may not occur in commercial products (Bauer and Wagner, 1991, Bauer, 1991). Only the polysaccharides (though not in high-alcohol extracts) and the alkylamides are currently accepted as active in commercial products.
COMMON OPINIONS FOR QUALITY ECHINACEA PRODUCTS
(according to leading herbalists and herbal manufacturers)
- The form (powder, liquid, etc.) is less important than the quality of the starting herb and the care taken in the manufacturing process, though most, if not all, of the clinical and laboratory tests and reports used a liquid alcoholic preparation.
- Entirely from certified organically grown plants (for the purpose of preserving wild populations, some of which are becoming endangered).
- The fresher the better. Extracts should ideally be made from the fresh plants.
- Products should contain at least one part of or a combination of the roots, leaves, and flowers of E. purpurea or this plus the roots of E. angustifolia.
- Products should be certified to contain only Echinacea purpurea or E. angustifolia (not the common adulterant Parthenium integrifolium or other species of Echinacea).
- If the product contains E. pallida (wild or cultivated) or E. tennesseensis (cultivated only!), this will be clearly noted.
- If the herb is extracted, the ideal menstruum is an ethanol to water mixture of 55/45% for the dried root, or about 75% for fresh roots or tops.
- Fresh juice preparations should be made from high-quality fresh plant material and properly stabilized with about 20-25% ethanol.
- Dried, powdered extracts should ideally be made from fresh material.
Some researchers have suggested that combination preparations containing more than one immuno-stimulating plant extract, such as Baptisia tinctoria and Thuja occidentalis, may be more effective than echinacea alone, because of a synergistic effect, but this has yet to be proven in direct comparative human studies (Wagner and Jurcic, 1991; Schranner, et al.).
In Europe, a number of products derived from cell cultures of Echinacea species are being developed and tested. These cultures may have future importance in the production of large quantities of standardized "phytomedicines" for use by physicians and some herbalists (Sícha, et al., 1989).
DOSAGE AND DURATION Of APPLICATION
According to Wagner and Jurcic, immune reactions work by the law of all or nothing; that is, when immune modulators (such as Echinacea products) reach a critical dose, they lead to an immune response, which can not be further increased by raising the concentration, and may even, in some cases, lead to immune suppression (Wagner and Jurcic, 1991).
Because preliminary tests have shown the activity of echinacea extracts to be dose-dependent, there has been a call to increase the quantity and quality of testing to determine the correct dose of echinacea for effective immune-stimulation (Gaisbauer, 1990b). This may be partly answered by the placebo-controlled, double-blind study (Bräunig, et al.) published in 1992, previously mentioned, which showed that 180 drops of an ethanolic extract of E. purpurea (roots) significantly reduced the severity and duration of the symptoms of flu-like infections, whereas 90 drops was no more effective than a placebo preparation (containing ethanol and sugar coloring).
In another study (Jurcic, et al., 1989), maximum immune stimulation was reached after 5 days, after which the immune stimulation began to subside, even though the preparation was continued at the same dosage.
ADULTERANTS
Echinacea is a plant remedy that has been commonly adulterated in the market throughout its commercial history. The most common adulterant now is Parthenium integrifolium, which is often sold as E. purpurea.
It has become evident through the work of Steven Foster (1985a, b), Rudolf Bauer (1987c), and others, that most of the commercially available root of Echinacea purpureaon the U.S. market was (before about 1988) in fact, Parthenium. Fortunately, many commercial product manufacturers have switched to certified organic herb in the last few years. Fresh E. purpurea products from above ground parts have not been affected by adulteration.
In 1987, a number of American manufacturers met under the auspices of the American Herbal Products Association (AHPA) and agreed to not sell Parthenium as Echinacea and to make Parthenium products labelled as such.
This adulteration is not new. Herb sellers have been substituting Parthenium, especially for E. purpurea, for well over 80 years, probably because the roots of Parthenium are much weightier and easier to harvest, and because populations of wild E. purpurea are scattered and sparse. In all likelihood, any commercial root products or bulk herb of E. purpurea that did not originate from a cultivated source are probably Parthenium integrifolium (Foster, 1990). John Uri Lloyd noted a "spurious adulterant" later identified as P. integrifolium in 1910 (J. Moser 1910).
Besides Parthenium, E. pallida is also sold as E. angustifolia (Bauer, 1991b), and this is rarely mentioned on product labels and in accompanying literature (author's observation). While this latter species may have immune-activating properties, and it was formerly official in the National Formulary, it has not undergone the extensive testing that the other two species have, and therefore should be clearly marked when it is included in commercial products.
Other plants that were formerly substituted for commercial Echinacea sp. include Lespedeza capitata (bush clover) and Eryngium aquaticum (eryngo) (Lloyd, 1917).
ANALYSIS OF THE CRUDE HERB AND COMMERCIAL PRODUCTS
Analysis of commercial supplies and products of echinacea is by visual inspection, taste, microscopic analysis, and chemical analysis by thin-layer chromatography (TLC) or high-performance liquid chromatography (HPLC). It has recently become obvious, through increased scrutiny and regulatory pressures, that insuring the proper identity and even quality of commercial supplies of echinacea (and other herbs as well) is essential if the herb industry is to continue to mature.
The following analytical methods can be consulted for identification/differentiation of the species. See also Bauer and Wagner (1990, 1991).
- Microscopic, botanical analysis: Kraemer and Sollenberger, 1911; Heubl, et al., 1988; Heubl and Bauer, 1990; Schulthess, et al., 1991 (achenes).
- TLC analysis: Bauer, et al., 1986, 1987
- HPLC analysis: Bauer, et al., 1987; Bauer et al., 1988e; Schulthess, et al., 1991 (identification and differentiation of the 3 main commercial species from the achenes).
STANDARDIZATION
Although some European products use echinacoside as a reference standard (author's observation), a study published in 1991 (Schumacher & Friedberg) has supported the previously held finding that this compound is not particularly active; also, it is not present in E. purpurea. Other active compounds, such as alkylamides, may be the best candidates for eventual standardization. Recently, a super-critical CO(2) extract of E. purpurea root said to contain about 50% alkylamides was claimed in a Japanese patent to be useful for inflammation, edema, and immune disorders (Etsuio Schulthess, et al., 1991 Enriko, 1992).
Wagner and Jurcic (1991) reported that the concentration for active low-molecular weight compounds is often in the range of 0.1 to 1%. High molecular-weight compounds (such as polysaccharides or glycoproteins) are difficult to isolate and characterize, but Esberitox(R) is now standardized to glycoproteins, and commercial products standardized to polysaccharides from cell cultures are under development.
CULTIVATION
Because of the rapidly dwindling supplies of wild plants, the commercial organic cultivation of echinacea is of extreme importance. It has been reported that over 100,000 pounds of wild echinacea have been harvested and shipped overseas for many years, and demand in the U.S. has dramatically increased since the mid-1980s. As the popularity of echinacea has increased over the last 10 years, the wild supplies are increasingly pressured, leading to over-harvesting and the harvesting of endangered or rare species. High-quality wild-crafted E. angustifolia has been increasingly difficult to find in the 1991-1993 seasons.
Fortunately, there are at least two large suppliers of certified-organically grown E. purpurea and to a lesser extent, E. angustifolia and E. pallida, though the latter plant is not as commercially popular as the first two. Based on projections for future demand, focus on echinacea cultivation by organic growers would seem commercially feasible (Blumenthal, 1993).
Specific cultivation techniques will not be included here, but thorough reviews are available (Hobbs, 1989; Foster, 1991).
TOXICOLOGY
Historical human use and experimental animal studies indicate little need for concern regarding the safety of echinacea. Some toxicological work on the toxicity of echinacea has been done, mainly on the fresh tops of Echinacea purpurea For a review of toxicological studies, see the additional information in Chemistry/Pharmacy.
PHARMACY AND PHARMACOGNOSY
COMMERCIAL PREPARATIONS
As immunomodulators, the popularity of echinacea preparations is increasing worldwide. For instance, in Germany (1989), the most popular echinacea product is 131st on a list of the 2,000 most-prescribed drugs (Schumacher & Friedberg, 1991), and is possibly the most popular herb in the U.S, with sales increasing dramatically over the last few years. It is obvious to the herbal consumer that there is an increasing array of echinacea products available in the natural food store or herb shop. The use of echinacea seems to "cross over" into groups of people who wouldn't normally use herbal products, and even Hollywood has discovered echinacea. It was recently reported in the popular press that celebreties such as Cher, Jodie Foster, and members of the Star Trek cast commonly use the herb to ward off colds and flus (Garstang, 1993). Much of this popularity seems to be due to simple word-of-mouth transmission.
At present, the most popular echinacea products are liquid extracts (made with varying proportions of grain alcohol and water, and, in some cases, glycerin), spray- or freeze-dried extracts in capsules and tablets, simple herb powders in capsules and tablets and the fresh juice of E. purpurea tops stabilized with ethanol.
Of the proven immuno-active compounds from Echinacea spp., the polyacetylenes and cichoric acid are unstable and may not occur in commercial products (Bauer and Wagner, 1991, Bauer, 1991). Only the polysaccharides (though not in high-alcohol extracts) and the alkylamides are currently accepted as active in commercial products.
COMMON OPINIONS FOR QUALITY ECHINACEA PRODUCTS
(according to leading herbalists and herbal manufacturers)
- The form (powder, liquid, etc.) is less important than the quality of the starting herb and the care taken in the manufacturing process, though most, if not all, of the clinical and laboratory tests and reports used a liquid alcoholic preparation.
- Entirely from certified organically grown plants (for the purpose of preserving wild populations, some of which are becoming endangered).
- The fresher the better. Extracts should ideally be made from the fresh plants.
- Products should contain at least one part of or a combination of the roots, leaves, and flowers of E. purpurea or this plus the roots of E. angustifolia.
- Products should be certified to contain only Echinacea purpurea or E. angustifolia (not the common adulterant Parthenium integrifolium or other species of Echinacea).
- If the product contains E. pallida (wild or cultivated) or E. tennesseensis (cultivated only!), this will be clearly noted.
- If the herb is extracted, the ideal menstruum is an ethanol to water mixture of 55/45% for the dried root, or about 75% for fresh roots or tops.
- Fresh juice preparations should be made from high-quality fresh plant material and properly stabilized with about 20-25% ethanol.
- Dried, powdered extracts should ideally be made from fresh material.
Some researchers have suggested that combination preparations containing more than one immuno-stimulating plant extract, such as Baptisia tinctoria and Thuja occidentalis, may be more effective than echinacea alone, because of a synergistic effect, but this has yet to be proven in direct comparative human studies (Wagner and Jurcic, 1991; Schranner, et al.).
In Europe, a number of products derived from cell cultures of Echinacea species are being developed and tested. These cultures may have future importance in the production of large quantities of standardized "phytomedicines" for use by physicians and some herbalists (Sícha, et al., 1989).
DOSAGE AND DURATION Of APPLICATION
According to Wagner and Jurcic, immune reactions work by the law of all or nothing; that is, when immune modulators (such as Echinacea products) reach a critical dose, they lead to an immune response, which can not be further increased by raising the concentration, and may even, in some cases, lead to immune suppression (Wagner and Jurcic, 1991).
Because preliminary tests have shown the activity of echinacea extracts to be dose-dependent, there has been a call to increase the quantity and quality of testing to determine the correct dose of echinacea for effective immune-stimulation (Gaisbauer, 1990b). This may be partly answered by the placebo-controlled, double-blind study (Bräunig, et al.) published in 1992, previously mentioned, which showed that 180 drops of an ethanolic extract of E. purpurea (roots) significantly reduced the severity and duration of the symptoms of flu-like infections, whereas 90 drops was no more effective than a placebo preparation (containing ethanol and sugar coloring).
In another study (Jurcic, et al., 1989), maximum immune stimulation was reached after 5 days, after which the immune stimulation began to subside, even though the preparation was continued at the same dosage.
ADULTERANTS
Echinacea is a plant remedy that has been commonly adulterated in the market throughout its commercial history. The most common adulterant now is Parthenium integrifolium, which is often sold as E. purpurea.
It has become evident through the work of Steven Foster (1985a, b), Rudolf Bauer (1987c), and others, that most of the commercially available root of Echinacea purpureaon the U.S. market was (before about 1988) in fact, Parthenium. Fortunately, many commercial product manufacturers have switched to certified organic herb in the last few years. Fresh E. purpurea products from above ground parts have not been affected by adulteration.
In 1987, a number of American manufacturers met under the auspices of the American Herbal Products Association (AHPA) and agreed to not sell Parthenium as Echinacea and to make Parthenium products labelled as such.
This adulteration is not new. Herb sellers have been substituting Parthenium, especially for E. purpurea, for well over 80 years, probably because the roots of Parthenium are much weightier and easier to harvest, and because populations of wild E. purpurea are scattered and sparse. In all likelihood, any commercial root products or bulk herb of E. purpurea that did not originate from a cultivated source are probably Parthenium integrifolium (Foster, 1990). John Uri Lloyd noted a "spurious adulterant" later identified as P. integrifolium in 1910 (J. Moser 1910).
Besides Parthenium, E. pallida is also sold as E. angustifolia (Bauer, 1991b), and this is rarely mentioned on product labels and in accompanying literature (author's observation). While this latter species may have immune-activating properties, and it was formerly official in the National Formulary, it has not undergone the extensive testing that the other two species have, and therefore should be clearly marked when it is included in commercial products.
Other plants that were formerly substituted for commercial Echinacea sp. include Lespedeza capitata (bush clover) and Eryngium aquaticum (eryngo) (Lloyd, 1917).
ANALYSIS OF THE CRUDE HERB AND COMMERCIAL PRODUCTS
Analysis of commercial supplies and products of echinacea is by visual inspection, taste, microscopic analysis, and chemical analysis by thin-layer chromatography (TLC) or high-performance liquid chromatography (HPLC). It has recently become obvious, through increased scrutiny and regulatory pressures, that insuring the proper identity and even quality of commercial supplies of echinacea (and other herbs as well) is essential if the herb industry is to continue to mature.
The following analytical methods can be consulted for identification/differentiation of the species. See also Bauer and Wagner (1990, 1991).
- Microscopic, botanical analysis: Kraemer and Sollenberger, 1911; Heubl, et al., 1988; Heubl and Bauer, 1990; Schulthess, et al., 1991 (achenes).
- TLC analysis: Bauer, et al., 1986, 1987
- HPLC analysis: Bauer, et al., 1987; Bauer et al., 1988e; Schulthess, et al., 1991 (identification and differentiation of the 3 main commercial species from the achenes).
STANDARDIZATION
Although some European products use echinacoside as a reference standard (author's observation), a study published in 1991 (Schumacher & Friedberg) has supported the previously held finding that this compound is not particularly active; also, it is not present in E. purpurea. Other active compounds, such as alkylamides, may be the best candidates for eventual standardization. Recently, a super-critical CO(2) extract of E. purpurea root said to contain about 50% alkylamides was claimed in a Japanese patent to be useful for inflammation, edema, and immune disorders (Etsuio Schulthess, et al., 1991 Enriko, 1992).
Wagner and Jurcic (1991) reported that the concentration for active low-molecular weight compounds is often in the range of 0.1 to 1%. High molecular-weight compounds (such as polysaccharides or glycoproteins) are difficult to isolate and characterize, but Esberitox(R) is now standardized to glycoproteins, and commercial products standardized to polysaccharides from cell cultures are under development.
CULTIVATION
Because of the rapidly dwindling supplies of wild plants, the commercial organic cultivation of echinacea is of extreme importance. It has been reported that over 100,000 pounds of wild echinacea have been harvested and shipped overseas for many years, and demand in the U.S. has dramatically increased since the mid-1980s. As the popularity of echinacea has increased over the last 10 years, the wild supplies are increasingly pressured, leading to over-harvesting and the harvesting of endangered or rare species. High-quality wild-crafted E. angustifolia has been increasingly difficult to find in the 1991-1993 seasons.
Fortunately, there are at least two large suppliers of certified-organically grown E. purpurea and to a lesser extent, E. angustifolia and E. pallida, though the latter plant is not as commercially popular as the first two. Based on projections for future demand, focus on echinacea cultivation by organic growers would seem commercially feasible (Blumenthal, 1993).
Specific cultivation techniques will not be included here, but thorough reviews are available (Hobbs, 1989; Foster, 1991). REFERENCES
Ammann, M. and K. Suter. 1987. Deut. Apoth. Zeit. 127: 853.
Auster, F. and J. Schafer. 1957. Echinacea angustifolia. VEB G. Thieme, Leipzig.
Baetgen, D. 1984. Therapiewoche 34: 5115.
Baetgen, D. 1988. T.W. Pädiatrie 1: 66.
Bauer, K. M. 1958. Landarzt 34: 5115-9.
Bauer, R. and H. Wagner. 1990. Echinacea, Wissenshaftliche. Stuttgart: Verlagsgesellschaft.
Bauer, R., P. Remiger, and E. Alstat. 1990b. Planta Med. 56: 533-4.
Bauer, R. and H. Wagner. 1991. In Economic and Medicinal Plant Research, v. 5. Wagner, H. & N. R. Farnsworth, eds. New York: Academic Press.
Bauer, R. 1991. Personal Communication.
Bauer, R. and S. Foster. 1991. Planta Med. 57: 447-9.
Bauer, R., P. Remiger, and H. Wagner. 1988a. Dtsch. Apoth. Ztg. 128: 174-80.
Bauer, R., P. Remiger, and H. Wagner. 1989. Phytochemistry 28: 505-8.
Bauer, R., P. Remiger, K. Jurcic, and H. Wagner. 1989. Z. Phytother. 10: 43-8.
Bauer, R. and P. Remiger. 1989b. Planta Med. 55: 367-71.
Bauer, R. and H. Wagner. 1987. Sci. Pharm. 55: 159-61.
Bauer, R., P. Remiger, V. Wray, and H. Wagner. 1988b. Planta Med. 54: 478-9.
Bauer, R., I. A. Khan, and H. Wagner. 1988e. Planta Med. 54: 426-30.
Bauer, R., K. Jurcic, J. Puhlmann,and H. Wagner. 1988d. Arzneim.-Forsch. 38: 276-81.
Bauer, R., I. A. Khan, and H. Wagner. 1986. Sci. Pharm. 54: 145.
Bauer, R. Personal communication, Jan. 19, 1993.
Becker, H. 1982. Deut. Apoth. Zeit. 122: 2320.
Becker, H., et al. 1985. Z. Naturforsch. 40c: 585-7.
Berkeley, E. and D. S. Berkeley. 1963. John Clayton, Pioneer of American Botany, University of North Carolina, p. 143.
Beuscher, H. and L. Kopanski. 1987. Pharm. Weekbl. Sci. Ed. 9: 329.
Bittner, E. 1969. Ph.D. Dissertation. Albert Ludwigs Universität, Freiburg.
Blake, A. K. 1929. New Asteraceae from the United States, Mexico and Honduras. Jour. Wash. Acad. Sci. 19: 273.
Blumenthal, M., personal communication, June 10, 1993.
Bohl, R. and T. Hermann. 1954. Schweiz med. Wschr. 84: 421.
Bohlmann, F. and M. Grenz. 1966. Chem. Ber. 99: 3197.
Bohlmann, F and H. Hoffmann. 1983. Phytochemistry 22: 1173.
Bonadeo, I.G., G. Botazzi, and M. Lavazza. 1971. Riv. Ital. Essenze 53: 281.
Bos, R., F. Heinzer, and R. Bauer. 1988. Poster, 19th International Symposium on Essential Oils and Other Natural Substrates. Zürich, 7-10 September.
Boshamer, K. 1968. Lehrbuch der Urologie. Gustav Fischer Verlag, p. 74.
Bräunig, B., B. Dorn, and E. M. Knick. 1992. Z. Phytother. 13: 7.
Brehm, G. 1962. Arztl. Sammelbl. 51: 423.
Bridger, Bobby. Personal communication with Mark Blumenthal.
Britton, N. L. and A. Brown. 1896. An Illustrated Flora of the Northern United States, Canada and the British Possesions. NY: Charles Scribner's Sons.
Büsing, K. H. 1952. Arzn.-Forsch. 2: 467.
Büsing, K.H. 1958. Z. Immunitätsforsch. Exp. Ther. 115: 169-76.
Büsing, K. H. and G. Thüigen. 1959. G. Allerg. Asthma 4: 30-3.
Chamberlin, C. S. 1905. Lancet-Clinic n.s. 54: 279.
Cheminat, A., R. Zawatzky, H. Becker, and R. Brouillard. 1988. Phytochemistry 27: 2787-94.
Cheminat, A., R. Brouillard, P. Guerne, P. Bergmann, and B. Rether. 1989. Phytochemistry 28: 3246-7.
Choné, B. 1969. Dt. Med. Woch. 94: 1406.
Clayton, J. and J. F. Gronovius. 1762. Flora Virginica, Leiden.
Coeugniet, E. G. 1987. Klin. Pharm. (8/87): 481-85.
Coeugniet, E. G. and E. Elek. 1987. Suppl. Z. Onkologie 10: 27-33.
Coeugniet, E. and R. Kühnast. 1986. Therapiewoche 36: 3352.
Comings, I. M. 1847. New. Engl. Bot. Med. Surg. J. 1: 41.
Couch, J. F. and T. Giltner. 1921a. Am. J. Pharm. 93: 227.
Couch, J. F. and T. Giltner. 1921b. Am. J. Pharm. 93: 324.
Culter, S. H. 1930. J. Am. Pharm. Assn. 19: 120.
DeCandolle, A. P. and A. DeCandolle. 1824-1873. Prodromus systematis naturalis regni vegetabilis. 17 v. Paris.
Dorn, M. 1989. Natur Ganzheits-Med. 2: 314.
Dress, W. J. 1961. Baileya 9: 67.
Editorial. 1930. J. Am. Pharm. Assn. 19: 370.
Egert, D. and N. Beuscher. 1992. Planta Med. 58: 163.
Eilmes, H. G. 1976. Dissertation, Frankfurt.
Etsuio, B. and F. Enriko. 1992. Jpn. Kokai Tokkyo Koho, 5 pp. (Therapeutic extracts of Echinacea purpurea).
Felder, H. 1959. Med. Kiln. 12: 525.
Fong, et al. 1972. Lloydia 35: 38.
Fontana, A. In Press. (through Schiedges, Madaus).
Foster, S. 1991. Echinacea, Nature's Immune Enhancer. Rochester, VT: Healing Arts Press.
Foster, S. 1985a. Business of Herbs 8.
Foster, S. 1985b. HerbalGram 3.
Franken, E. and N. Sönnichsen. 1966. Aesthet. Medizin. (8/66): 242-45.
Freyer, H. U. 1974. Forschrift. der Ther. 92: 165.
Gaertner, W. 1963. Landarzt. 39: 123.
Gaisbauer, M. 1990a. Natura Med. 5: 176-90.
Gaisbauer, M. and T. Schleich. 1986. Natura Med. 1: 6.
Gaisbauer, M., T. Schleich, H. A. Stickl, and I. Wilczek. 1990b. Arzneim.-Forsch. 40: 594-8.
Gaisbauer, M. 1990. Natura Med. 5: 176-90.
Gartang, E. 1993. National Examiner, January 12, p. 7.
Gasiorowska, I., et al. 1981. Czas. Stomat. 34: 677.
Geller, C. A. 1987. Clinical experience, personal communication.
Giesbert, M. 1943. Fschr. Ther. 19: 4.
Giger, E. and T.W. Baumann. 1989. Planta Med. 55: 638.
Gilmore, M. R. 1911. Bur. Am. Eth. Ann. Rep. 33, p. 368.
Gilmore, M. R. 1913. Neb. St. Hist. Soc., Coll. 17: 332.
Gray, A. 1848. Manual of Botany. Boston: James Munroe and Co.
Gray, A. 1879. School and Field Book of Botany. NY: Iveson, Blakeman, Taylor, p. 205.
Greger, H. 1988. In Chemistry and Biology of Naturally Occurring Acetylenes and Related Compounds. J. Lam, H. Breteler, T. Arnason and L. Hansen, eds., pp. 159-78. Amsterdam: Elsevier.
Gunther, E., E. F. Heeger, C. Rosenthal. 1952. Pharmazie 7: 24.
Hanfstaengel, E. and H. Ranz. 1956. Zbl. Chir., p. 2549.
Hansen, P. 1965. Prophylaxe 4: 278.
Heesen, W. 1964. Ehk 13: 209.
Heinzer, F., M. Chavanne., J.-P. Meusy, H.-P. Maitre, E. Giger,. and T.W. Baumann. 1988. Pharm. Acta. Helv. 63: 132-36.
Hentges, D. J. 1983. Human Intestinal Microflora in Health and Disease. New York: Academic Press.
Herrmann, G. 1952. Münch. med. Wschr. 94: 385.
Heubl, G. R. and R. Bauer. 1988. Sci. Pharm. 56: 145-60.
Heubl, G. R. & R. Bauer. 1990. Dtsch. Apoth. Ztg. 129: 2497-99.
Heyl, F. W. and M. C. Hart. 1915. J. Am. Chem. Soc. 37: 1769.
Heyl, F. W. and J. F. Staley. 1914. Am. J. Pharm. 86: 450.
Hobbs, C. R. 1989. The Echinacea Handbook. Capitola: Botanica Press.
Hoh, K. 1990. Ph. D. Dissertation, Freiburg.
Höreth, W. and F. Heiss. 1957. Medizinische, p. 297.
Hunsdorfer, N. W. 1954. Ärztl. Praxis IV/8:11.
Jacobson, M. 1954. Science 120: 1028.
Jacobson, M. 1967. J. Org. Chem. 32: 1646.
Jacobson, M. 1975. Lloydia 38: 473.
Jurcic, K., D. Melchart, M. Holzmann, P. Martin, R. Bauer, A. Doenicke, and H. Wagner. 1989. Z. Phytother. 10: 67-70.
Kelsey, H. P. and W. A. Dayton. 1942. Standardized Plant Names. Harrisburg: McFarland Co.
Kennelly, J. C. 1985a. Microtest Research Ltd.: York, England.
Kennelly, J. C. 1985b. Microtest Research Ltd.: York, England.
King, J. & H. C. F. Meyer. 1887. Eccl. Med. J. 48: 209.
King, J. 1852. The American Dispensatory. Cincinnati: H.W. Derby.
Kinkel, H. J., M. Plate, and H. U. Tullner. 1984. Med. Klin. 79: 580.
Kleinschmidt, H. 1965. Ther. d. Gegen. p. 1258.
Koch, Fr. E. 1953. Arzn.-Forsch. 3: 16.
Koch, Fr. E. 1952. Arzn.-Forsch. 2: 464.
Korting, G. W. and K. Rasp. 1954. Medizinische 45: 1504.
Kraemer, H. and M. Sollenberger. 1911. Am. J. Pharm. 83: 315.
Krause, M. 1984. Dissertation, Berlin.
Krause, W. 1986. Dissertation, Tübingen.
Kuhn, O. 1953. Arzn.-Forsch. 3: 194.
Lang, W. and U. Mengs. 1976a. Report on Echinacea Toxicity in Mice. Cologne: Madaus GMBH.
Lang, W. and U. Mengs. 1976b. Report on Echinacea Toxicity in Rats. Cologne: Madaus GMBH., September 1, 1976.
Lasch, H. G. 1983. Die Med. Welt. 34: 1463.
Leng-Peschlow, E. In press. (through Schiedges, Madaus).
Lenk, W. 1989. Z. Phytother. 10: 49-51.
Lersch, C., et al. 1990a. J. Exp. Clin. Cancer Res. 9: 247-50.
Lersch, C., et al. 1990b. Arch. Geschwulstforsch. 60: 379-83.
Lersch, C., et al. 1992. Tumordiagn. u. Ther. 13: 115-20.
Luettig, B., C. Steinmüller, G. E. Gifford, H. Wagner, and M.-L. Lohmann-Matthes. 1989. J. Natl. Cancer Inst. 81: 669-75.
Linnaeus, C. 1753. Species Plantarum, Vol. 2, London: Bernard Quaritch (1959), p. 907.
Lloyd, J. U. 1897. Eccl. Med. J. 57: 28.
Lloyd, J. U. 1904. Pharm. Rev. 22: 9. Reprinted by the American Herb Association, Rescue, CA.
Lloyd, J. U. 1917. A Treatise on Echinacea. Cincinnati: Lloyd Bros.; reprinted by Herb-Pharm, Williams, OR.
Lohmann-Matthes, M.-L., and H. Wagner. 1989. Z. Phytother. 10: 52-9.
Lücker, P. W. 1982. The Stimulant Value of Intravenous Application of Echinacin(R). Institut für Klinische Pharmakologie, Bobenheim am Berg.
Lyons, A.B. 1907. Plant Names, Scientific and Popular, 2nd ed. Detroit: Nelson, Baker & Co.
Malonga-Makosi J.-P. 1983. Dissertation, University of Heidelberg.
Martin, R. 1985. Dissertation, University of Heidelberg.
May, G. and G. Willuhn. 1978. Arzn.-Forsch. 28: 1, 2242.
McGregor, R. L. 1968. Univ. of Kansas Sci. Bul. 48: 132.
Meixner, H. K. L. 1953. Med. heute 11: 314.
Mengs, U. 1985. Biologisches Institut Dr. Madaus GMBH & Co., Cologne.
Mengs, U., C. B. Clare, and J. A. Poiley. 1991. Arzneim.-Forsch. 41: 1076-81.
Miller, K. In press. (through Schiedges, Madaus).
Moench, K. 1794. Methodas Plantas.
Möller, H. & H. Naumann. 1987. Therapeutikon 1: 56-61.
Morison, R. 1699. Plantarum Historiae Universalis Oxoniensis, Pars tertia finished and published by Jacob Bobart the younger, p. 42.
Möse, J. R. 1983. Die Mediz. Welt. 34: 1463.
Moser, J. 1910. Am. J. Pharm. 82: 224.
Mostbeck, A. and Studlar, M. 1962. Wiener Med. Wschr. 112: 259.
Mund-Hoym, W.-D. 1979. Arztl. Prax. 31: 566.
Neugebauer, H. 1949. Pharmazie 4: 137.
Norton, J. B. 1902. Notes on some plants of the Southwestern United States. Trans. Acad. Sci. St. Louis 7: 40-41.
Nuttall, T. 1834. J. Acad. Nat. Sci. of Phil. 7: 77.
Nuttall, T. 1841. Trans. Am. Phil. Soc. n.ser. 7: 354.
Pohl, P. 1969. Med. Klin. 35: 1546.
Quadripur, S.-A. 1976a. Medikamentose Beein. d. Phag. Gran. 115: 1072.
Quadripur, S.-A. 1976b. Ther. d. Gegen. 115: 1072.
Rafinesque, C. S. 1830. Medical Botany. Philadelphia: Samuel Atkinson, p. 227.
Reissmann, G. 1966. Folia haemotologica 85: 125.
Reith, F. J. 1978. Patent Ger. Offen 2: 721: 014 through CA 90:43820t.
Remiger, P. Dissertation, University of Munich.
Reuss, D. 1981. Z Allgemeinmed 57: 865.
Reuss, D. 1986. Rheuma 5:29-32.
Riddell, J. L. 1835/6. A Synopsis of the Flora of the Western States, p. 58.
Robinson, H. 1978. Phytologia 41: 39-44.
Röder, E., et al. 1984. Deut. Apoth. Zeit. 124: 2316.
Röseler, W. 1952. Medizinische : 93.
Rösler, J., C. Steinmüller, A. Kinderlen, A. Emmendörffer, H. Wagner, and M.-L. Lohmann-Matthes. 1991a. Int. J. Immunopharmacol. 13: 27-31.
Rösler, J., Emmendörffer, Steinmüller, Luettig, B., Wagner, H. and M.-L. Lohmann-Matthes. 1991b. Int. J. Immunopharmac. 13: 931-41.
Sartor, K. J. 1972. Ther. d. Gegenw.: 1147.
Schiedges, K. L. Madaus AG. Personal communication, May 26, 1992.
Schimmel, K. Ch., and G. T. Werner. 1981. Ther. d. Gegen. 120: 1065.
Schimmer, O., et al. 1989. Z. Phytother. 10: 39-42. Schindler, H. 1953. Arzn.-Forsch. 3: 485.
Schmidt, U., M. Albrecht, and N. Schenk. 1990. Natur Ganzheits-Med. 3: 277.
Schöneberger, D. 1992. Zeit. f. Immunologic Praxis (Forum Immunologic 8: 2-12.).
Schranner, I., Würdinger, N. Klumpp, U. Lösch and S. N. Okpanyi. 1989. J. Vet. Med. B 36: 353-64.
Schulte, K. E., G. Ruecker, and J. Perlick. 1967. Arzn.-Forsch. 17: 825-29.
Schulthess, B. H., E. R. Giger, and T. W. Baumann. 1988. Poster, 36th Annual Congress of the Society of Medicinal Plant Research, Freiburg, 12-16 September.
Schulthess, B. H., E. Giger, and T.W. Baumann. 1991. Planta Med. 57: 384-8.
Schumacher, A. and K.-K. Friedberg. 1991. Arzneim.-Forsch. 41: 141-7.
Schuster, A. 1952. Med. Mschr. 6: 453.
Sícha, J., J. Hubík, and J. Dusek. 1989. Ceskoslov. Farm. 38: 128-9.
Sícha, J., H. Becker, J. Dusek, T. Hubík, J. Siatka and I. Hrones. 1991. Pharmazie 46: 363-4.
Sickel, K. 1971. Arztl. Prax. 23: 201.
Small, J. K. 1933. Manual of the Southeastern Flora. Chapel Hill: Univ. of N.C. Press.
Soicke, H., K. Görler, and D. Krüger. 1988. Fitoterapiea 59: 73-5.
Sprockhoff, O. 1964. Landarzt 40: 1173.
Sprockhoff, O. 1986. Ärztezeit. f. Naturheil. 27: 780.
Stimpel, M., A. Proksch, H. Wagner, and M.L. Lohmann-Matthes. 1984. Inf. and Immun. 46: 845.
Stites, D. P., J. D. Stobo, H. H. Fudenberg, and J.V. Wells. 1982. Basic and Clinical Immunology (4th ed.). Los Altos, CA: Lange Medical Publications.
Stoll, A., J. Renz and A. Brack. 1950. Helv. Chim. Acta 33: 1877.
Stotzem, C. D., U. Hungerland. and U. Mengs. 1992. Med. Sci. Res. 20: 719.
Stuessy, T F. 1977. In The Biology and Chemistry of the Compositae ed. V. H. Harborne, J.B. Harborne and B. L. Turner, Vol. II, pp. 622-671. London: Academic Press.
Stuppner, H. 1985. Ph.D. Dissertation, Ludwig-Maximillians-Universität, München.
Thackeray, W. T. 1923. Am. J. Clin. Med. 30: 430.
Tosetti, K. 1961. Dtsch. Gesuhdb.-Wes. 16: 64.
Tronnier, H. 1967. Munch. med. Wschr. 109: 2118.
Tubaro, A., et al. 1987. J. Pharm. Pharmacol. 39: 567.
Tympner, K.-D. 1978. Münch. med. Wschr. 120: 1055.
Tympner, K.-D., P. K. Klose and R. B. Pelka. 1987. Natura Med. 2: 78-84.
Uhlmann, W.-J. 1958. Medizinische 2: 81-4.
Unruh, V. 1915. Nat. Eclec. Med. Assn. Q. 7: 63.
Verelis, C. and H. Becker. 1977. Planta Med. 31: 288.
Verelis, C. 1978. Dissertation, University of Heidelberg.
Viehmann, P. 1978. Erhahrungsheilkunde 27: 353.
Voaden, D.J. and M. Jacobson. 1972. J. Med. Chem. 15: 619.
Vogel, G. et al. 1968. Arzn.-Forsch. 18: 426.
Vogel V. 1977. American Indian Medicine, Norman, Oklahoma. University of Oklahoma Press.
Volz, G. 1957. Ther. Ggw. 96: 312.
Vömel, T. 1985. Arzn.-Forsch. 35: 1437.
Vorberg, G. and B. Schneider. 1989. Ärztl. Fortschr. 36: 3.
Wacker, A., et al. 1973. Arzneim.-Forsch. 23: 119.
Wacker, A. and A. Hilbig. 1978. Planta Med. 33: 89.
Wagner, H., H. Stuppner, J. Puhlmann, B. Brümmer, K. Deppe, and M.A. Zenk. 1989. Z. Phytother. 10: 35-8.
Wagner, H., A. Proksch, et al. 1981. Z. für Angewandte Phytotherapie 2: 166.
Wagner, H and A. Proksch. 1985a. In: Economic and Medicinal Plant Research. Orlando: Academic Press, p. 113.
Wagner, H. and K. Jurcic. 1991. Arzn.-Forsch. 41: 1072-6.
Webster, H. T. 1891. Massachusetts Medical J. 11: 344.
Wedel, W. 1936. Bur. Am. Ethn. Bull. 112: 59.
Weissbach, L., et al. 1977. Therapiewoche 27: 6009.
Wember, S. 1953. Landarzt. 29: 621.
Wood -. 1925. Propagateur de l'Homeopathie.
Woods, E. L. 1930. Am. J. Pharm. 102: 611-630.
Zimmermann, O. 1969. Hippokrates 40: 233.
Some references mentioned in the text pertain to material on the chemistry and pharmacology of Echinacea originally included in this piece. Space restrictions prevented inclusion here, but the material is presented in a supplementary publication, "Echinacea :Chemistry and Pharmacology."
Article copyright American Botanical Council.
~~~~~~~~
By Christopher Hobbs
REFERENCES
Ammann, M. and K. Suter. 1987. Deut. Apoth. Zeit. 127: 853.
Auster, F. and J. Schafer. 1957. Echinacea angustifolia. VEB G. Thieme, Leipzig.
Baetgen, D. 1984. Therapiewoche 34: 5115.
Baetgen, D. 1988. T.W. Pädiatrie 1: 66.
Bauer, K. M. 1958. Landarzt 34: 5115-9.
Bauer, R. and H. Wagner. 1990. Echinacea, Wissenshaftliche. Stuttgart: Verlagsgesellschaft.
Bauer, R., P. Remiger, and E. Alstat. 1990b. Planta Med. 56: 533-4.
Bauer, R. and H. Wagner. 1991. In Economic and Medicinal Plant Research, v. 5. Wagner, H. & N. R. Farnsworth, eds. New York: Academic Press.
Bauer, R. 1991. Personal Communication.
Bauer, R. and S. Foster. 1991. Planta Med. 57: 447-9.
Bauer, R., P. Remiger, and H. Wagner. 1988a. Dtsch. Apoth. Ztg. 128: 174-80.
Bauer, R., P. Remiger, and H. Wagner. 1989. Phytochemistry 28: 505-8.
Bauer, R., P. Remiger, K. Jurcic, and H. Wagner. 1989. Z. Phytother. 10: 43-8.
Bauer, R. and P. Remiger. 1989b. Planta Med. 55: 367-71.
Bauer, R. and H. Wagner. 1987. Sci. Pharm. 55: 159-61.
Bauer, R., P. Remiger, V. Wray, and H. Wagner. 1988b. Planta Med. 54: 478-9.
Bauer, R., I. A. Khan, and H. Wagner. 1988e. Planta Med. 54: 426-30.
Bauer, R., K. Jurcic, J. Puhlmann,and H. Wagner. 1988d. Arzneim.-Forsch. 38: 276-81.
Bauer, R., I. A. Khan, and H. Wagner. 1986. Sci. Pharm. 54: 145.
Bauer, R. Personal communication, Jan. 19, 1993.
Becker, H. 1982. Deut. Apoth. Zeit. 122: 2320.
Becker, H., et al. 1985. Z. Naturforsch. 40c: 585-7.
Berkeley, E. and D. S. Berkeley. 1963. John Clayton, Pioneer of American Botany, University of North Carolina, p. 143.
Beuscher, H. and L. Kopanski. 1987. Pharm. Weekbl. Sci. Ed. 9: 329.
Bittner, E. 1969. Ph.D. Dissertation. Albert Ludwigs Universität, Freiburg.
Blake, A. K. 1929. New Asteraceae from the United States, Mexico and Honduras. Jour. Wash. Acad. Sci. 19: 273.
Blumenthal, M., personal communication, June 10, 1993.
Bohl, R. and T. Hermann. 1954. Schweiz med. Wschr. 84: 421.
Bohlmann, F. and M. Grenz. 1966. Chem. Ber. 99: 3197.
Bohlmann, F and H. Hoffmann. 1983. Phytochemistry 22: 1173.
Bonadeo, I.G., G. Botazzi, and M. Lavazza. 1971. Riv. Ital. Essenze 53: 281.
Bos, R., F. Heinzer, and R. Bauer. 1988. Poster, 19th International Symposium on Essential Oils and Other Natural Substrates. Zürich, 7-10 September.
Boshamer, K. 1968. Lehrbuch der Urologie. Gustav Fischer Verlag, p. 74.
Bräunig, B., B. Dorn, and E. M. Knick. 1992. Z. Phytother. 13: 7.
Brehm, G. 1962. Arztl. Sammelbl. 51: 423.
Bridger, Bobby. Personal communication with Mark Blumenthal.
Britton, N. L. and A. Brown. 1896. An Illustrated Flora of the Northern United States, Canada and the British Possesions. NY: Charles Scribner's Sons.
Büsing, K. H. 1952. Arzn.-Forsch. 2: 467.
Büsing, K.H. 1958. Z. Immunitätsforsch. Exp. Ther. 115: 169-76.
Büsing, K. H. and G. Thüigen. 1959. G. Allerg. Asthma 4: 30-3.
Chamberlin, C. S. 1905. Lancet-Clinic n.s. 54: 279.
Cheminat, A., R. Zawatzky, H. Becker, and R. Brouillard. 1988. Phytochemistry 27: 2787-94.
Cheminat, A., R. Brouillard, P. Guerne, P. Bergmann, and B. Rether. 1989. Phytochemistry 28: 3246-7.
Choné, B. 1969. Dt. Med. Woch. 94: 1406.
Clayton, J. and J. F. Gronovius. 1762. Flora Virginica, Leiden.
Coeugniet, E. G. 1987. Klin. Pharm. (8/87): 481-85.
Coeugniet, E. G. and E. Elek. 1987. Suppl. Z. Onkologie 10: 27-33.
Coeugniet, E. and R. Kühnast. 1986. Therapiewoche 36: 3352.
Comings, I. M. 1847. New. Engl. Bot. Med. Surg. J. 1: 41.
Couch, J. F. and T. Giltner. 1921a. Am. J. Pharm. 93: 227.
Couch, J. F. and T. Giltner. 1921b. Am. J. Pharm. 93: 324.
Culter, S. H. 1930. J. Am. Pharm. Assn. 19: 120.
DeCandolle, A. P. and A. DeCandolle. 1824-1873. Prodromus systematis naturalis regni vegetabilis. 17 v. Paris.
Dorn, M. 1989. Natur Ganzheits-Med. 2: 314.
Dress, W. J. 1961. Baileya 9: 67.
Editorial. 1930. J. Am. Pharm. Assn. 19: 370.
Egert, D. and N. Beuscher. 1992. Planta Med. 58: 163.
Eilmes, H. G. 1976. Dissertation, Frankfurt.
Etsuio, B. and F. Enriko. 1992. Jpn. Kokai Tokkyo Koho, 5 pp. (Therapeutic extracts of Echinacea purpurea).
Felder, H. 1959. Med. Kiln. 12: 525.
Fong, et al. 1972. Lloydia 35: 38.
Fontana, A. In Press. (through Schiedges, Madaus).
Foster, S. 1991. Echinacea, Nature's Immune Enhancer. Rochester, VT: Healing Arts Press.
Foster, S. 1985a. Business of Herbs 8.
Foster, S. 1985b. HerbalGram 3.
Franken, E. and N. Sönnichsen. 1966. Aesthet. Medizin. (8/66): 242-45.
Freyer, H. U. 1974. Forschrift. der Ther. 92: 165.
Gaertner, W. 1963. Landarzt. 39: 123.
Gaisbauer, M. 1990a. Natura Med. 5: 176-90.
Gaisbauer, M. and T. Schleich. 1986. Natura Med. 1: 6.
Gaisbauer, M., T. Schleich, H. A. Stickl, and I. Wilczek. 1990b. Arzneim.-Forsch. 40: 594-8.
Gaisbauer, M. 1990. Natura Med. 5: 176-90.
Gartang, E. 1993. National Examiner, January 12, p. 7.
Gasiorowska, I., et al. 1981. Czas. Stomat. 34: 677.
Geller, C. A. 1987. Clinical experience, personal communication.
Giesbert, M. 1943. Fschr. Ther. 19: 4.
Giger, E. and T.W. Baumann. 1989. Planta Med. 55: 638.
Gilmore, M. R. 1911. Bur. Am. Eth. Ann. Rep. 33, p. 368.
Gilmore, M. R. 1913. Neb. St. Hist. Soc., Coll. 17: 332.
Gray, A. 1848. Manual of Botany. Boston: James Munroe and Co.
Gray, A. 1879. School and Field Book of Botany. NY: Iveson, Blakeman, Taylor, p. 205.
Greger, H. 1988. In Chemistry and Biology of Naturally Occurring Acetylenes and Related Compounds. J. Lam, H. Breteler, T. Arnason and L. Hansen, eds., pp. 159-78. Amsterdam: Elsevier.
Gunther, E., E. F. Heeger, C. Rosenthal. 1952. Pharmazie 7: 24.
Hanfstaengel, E. and H. Ranz. 1956. Zbl. Chir., p. 2549.
Hansen, P. 1965. Prophylaxe 4: 278.
Heesen, W. 1964. Ehk 13: 209.
Heinzer, F., M. Chavanne., J.-P. Meusy, H.-P. Maitre, E. Giger,. and T.W. Baumann. 1988. Pharm. Acta. Helv. 63: 132-36.
Hentges, D. J. 1983. Human Intestinal Microflora in Health and Disease. New York: Academic Press.
Herrmann, G. 1952. Münch. med. Wschr. 94: 385.
Heubl, G. R. and R. Bauer. 1988. Sci. Pharm. 56: 145-60.
Heubl, G. R. & R. Bauer. 1990. Dtsch. Apoth. Ztg. 129: 2497-99.
Heyl, F. W. and M. C. Hart. 1915. J. Am. Chem. Soc. 37: 1769.
Heyl, F. W. and J. F. Staley. 1914. Am. J. Pharm. 86: 450.
Hobbs, C. R. 1989. The Echinacea Handbook. Capitola: Botanica Press.
Hoh, K. 1990. Ph. D. Dissertation, Freiburg.
Höreth, W. and F. Heiss. 1957. Medizinische, p. 297.
Hunsdorfer, N. W. 1954. Ärztl. Praxis IV/8:11.
Jacobson, M. 1954. Science 120: 1028.
Jacobson, M. 1967. J. Org. Chem. 32: 1646.
Jacobson, M. 1975. Lloydia 38: 473.
Jurcic, K., D. Melchart, M. Holzmann, P. Martin, R. Bauer, A. Doenicke, and H. Wagner. 1989. Z. Phytother. 10: 67-70.
Kelsey, H. P. and W. A. Dayton. 1942. Standardized Plant Names. Harrisburg: McFarland Co.
Kennelly, J. C. 1985a. Microtest Research Ltd.: York, England.
Kennelly, J. C. 1985b. Microtest Research Ltd.: York, England.
King, J. & H. C. F. Meyer. 1887. Eccl. Med. J. 48: 209.
King, J. 1852. The American Dispensatory. Cincinnati: H.W. Derby.
Kinkel, H. J., M. Plate, and H. U. Tullner. 1984. Med. Klin. 79: 580.
Kleinschmidt, H. 1965. Ther. d. Gegen. p. 1258.
Koch, Fr. E. 1953. Arzn.-Forsch. 3: 16.
Koch, Fr. E. 1952. Arzn.-Forsch. 2: 464.
Korting, G. W. and K. Rasp. 1954. Medizinische 45: 1504.
Kraemer, H. and M. Sollenberger. 1911. Am. J. Pharm. 83: 315.
Krause, M. 1984. Dissertation, Berlin.
Krause, W. 1986. Dissertation, Tübingen.
Kuhn, O. 1953. Arzn.-Forsch. 3: 194.
Lang, W. and U. Mengs. 1976a. Report on Echinacea Toxicity in Mice. Cologne: Madaus GMBH.
Lang, W. and U. Mengs. 1976b. Report on Echinacea Toxicity in Rats. Cologne: Madaus GMBH., September 1, 1976.
Lasch, H. G. 1983. Die Med. Welt. 34: 1463.
Leng-Peschlow, E. In press. (through Schiedges, Madaus).
Lenk, W. 1989. Z. Phytother. 10: 49-51.
Lersch, C., et al. 1990a. J. Exp. Clin. Cancer Res. 9: 247-50.
Lersch, C., et al. 1990b. Arch. Geschwulstforsch. 60: 379-83.
Lersch, C., et al. 1992. Tumordiagn. u. Ther. 13: 115-20.
Luettig, B., C. Steinmüller, G. E. Gifford, H. Wagner, and M.-L. Lohmann-Matthes. 1989. J. Natl. Cancer Inst. 81: 669-75.
Linnaeus, C. 1753. Species Plantarum, Vol. 2, London: Bernard Quaritch (1959), p. 907.
Lloyd, J. U. 1897. Eccl. Med. J. 57: 28.
Lloyd, J. U. 1904. Pharm. Rev. 22: 9. Reprinted by the American Herb Association, Rescue, CA.
Lloyd, J. U. 1917. A Treatise on Echinacea. Cincinnati: Lloyd Bros.; reprinted by Herb-Pharm, Williams, OR.
Lohmann-Matthes, M.-L., and H. Wagner. 1989. Z. Phytother. 10: 52-9.
Lücker, P. W. 1982. The Stimulant Value of Intravenous Application of Echinacin(R). Institut für Klinische Pharmakologie, Bobenheim am Berg.
Lyons, A.B. 1907. Plant Names, Scientific and Popular, 2nd ed. Detroit: Nelson, Baker & Co.
Malonga-Makosi J.-P. 1983. Dissertation, University of Heidelberg.
Martin, R. 1985. Dissertation, University of Heidelberg.
May, G. and G. Willuhn. 1978. Arzn.-Forsch. 28: 1, 2242.
McGregor, R. L. 1968. Univ. of Kansas Sci. Bul. 48: 132.
Meixner, H. K. L. 1953. Med. heute 11: 314.
Mengs, U. 1985. Biologisches Institut Dr. Madaus GMBH & Co., Cologne.
Mengs, U., C. B. Clare, and J. A. Poiley. 1991. Arzneim.-Forsch. 41: 1076-81.
Miller, K. In press. (through Schiedges, Madaus).
Moench, K. 1794. Methodas Plantas.
Möller, H. & H. Naumann. 1987. Therapeutikon 1: 56-61.
Morison, R. 1699. Plantarum Historiae Universalis Oxoniensis, Pars tertia finished and published by Jacob Bobart the younger, p. 42.
Möse, J. R. 1983. Die Mediz. Welt. 34: 1463.
Moser, J. 1910. Am. J. Pharm. 82: 224.
Mostbeck, A. and Studlar, M. 1962. Wiener Med. Wschr. 112: 259.
Mund-Hoym, W.-D. 1979. Arztl. Prax. 31: 566.
Neugebauer, H. 1949. Pharmazie 4: 137.
Norton, J. B. 1902. Notes on some plants of the Southwestern United States. Trans. Acad. Sci. St. Louis 7: 40-41.
Nuttall, T. 1834. J. Acad. Nat. Sci. of Phil. 7: 77.
Nuttall, T. 1841. Trans. Am. Phil. Soc. n.ser. 7: 354.
Pohl, P. 1969. Med. Klin. 35: 1546.
Quadripur, S.-A. 1976a. Medikamentose Beein. d. Phag. Gran. 115: 1072.
Quadripur, S.-A. 1976b. Ther. d. Gegen. 115: 1072.
Rafinesque, C. S. 1830. Medical Botany. Philadelphia: Samuel Atkinson, p. 227.
Reissmann, G. 1966. Folia haemotologica 85: 125.
Reith, F. J. 1978. Patent Ger. Offen 2: 721: 014 through CA 90:43820t.
Remiger, P. Dissertation, University of Munich.
Reuss, D. 1981. Z Allgemeinmed 57: 865.
Reuss, D. 1986. Rheuma 5:29-32.
Riddell, J. L. 1835/6. A Synopsis of the Flora of the Western States, p. 58.
Robinson, H. 1978. Phytologia 41: 39-44.
Röder, E., et al. 1984. Deut. Apoth. Zeit. 124: 2316.
Röseler, W. 1952. Medizinische : 93.
Rösler, J., C. Steinmüller, A. Kinderlen, A. Emmendörffer, H. Wagner, and M.-L. Lohmann-Matthes. 1991a. Int. J. Immunopharmacol. 13: 27-31.
Rösler, J., Emmendörffer, Steinmüller, Luettig, B., Wagner, H. and M.-L. Lohmann-Matthes. 1991b. Int. J. Immunopharmac. 13: 931-41.
Sartor, K. J. 1972. Ther. d. Gegenw.: 1147.
Schiedges, K. L. Madaus AG. Personal communication, May 26, 1992.
Schimmel, K. Ch., and G. T. Werner. 1981. Ther. d. Gegen. 120: 1065.
Schimmer, O., et al. 1989. Z. Phytother. 10: 39-42. Schindler, H. 1953. Arzn.-Forsch. 3: 485.
Schmidt, U., M. Albrecht, and N. Schenk. 1990. Natur Ganzheits-Med. 3: 277.
Schöneberger, D. 1992. Zeit. f. Immunologic Praxis (Forum Immunologic 8: 2-12.).
Schranner, I., Würdinger, N. Klumpp, U. Lösch and S. N. Okpanyi. 1989. J. Vet. Med. B 36: 353-64.
Schulte, K. E., G. Ruecker, and J. Perlick. 1967. Arzn.-Forsch. 17: 825-29.
Schulthess, B. H., E. R. Giger, and T. W. Baumann. 1988. Poster, 36th Annual Congress of the Society of Medicinal Plant Research, Freiburg, 12-16 September.
Schulthess, B. H., E. Giger, and T.W. Baumann. 1991. Planta Med. 57: 384-8.
Schumacher, A. and K.-K. Friedberg. 1991. Arzneim.-Forsch. 41: 141-7.
Schuster, A. 1952. Med. Mschr. 6: 453.
Sícha, J., J. Hubík, and J. Dusek. 1989. Ceskoslov. Farm. 38: 128-9.
Sícha, J., H. Becker, J. Dusek, T. Hubík, J. Siatka and I. Hrones. 1991. Pharmazie 46: 363-4.
Sickel, K. 1971. Arztl. Prax. 23: 201.
Small, J. K. 1933. Manual of the Southeastern Flora. Chapel Hill: Univ. of N.C. Press.
Soicke, H., K. Görler, and D. Krüger. 1988. Fitoterapiea 59: 73-5.
Sprockhoff, O. 1964. Landarzt 40: 1173.
Sprockhoff, O. 1986. Ärztezeit. f. Naturheil. 27: 780.
Stimpel, M., A. Proksch, H. Wagner, and M.L. Lohmann-Matthes. 1984. Inf. and Immun. 46: 845.
Stites, D. P., J. D. Stobo, H. H. Fudenberg, and J.V. Wells. 1982. Basic and Clinical Immunology (4th ed.). Los Altos, CA: Lange Medical Publications.
Stoll, A., J. Renz and A. Brack. 1950. Helv. Chim. Acta 33: 1877.
Stotzem, C. D., U. Hungerland. and U. Mengs. 1992. Med. Sci. Res. 20: 719.
Stuessy, T F. 1977. In The Biology and Chemistry of the Compositae ed. V. H. Harborne, J.B. Harborne and B. L. Turner, Vol. II, pp. 622-671. London: Academic Press.
Stuppner, H. 1985. Ph.D. Dissertation, Ludwig-Maximillians-Universität, München.
Thackeray, W. T. 1923. Am. J. Clin. Med. 30: 430.
Tosetti, K. 1961. Dtsch. Gesuhdb.-Wes. 16: 64.
Tronnier, H. 1967. Munch. med. Wschr. 109: 2118.
Tubaro, A., et al. 1987. J. Pharm. Pharmacol. 39: 567.
Tympner, K.-D. 1978. Münch. med. Wschr. 120: 1055.
Tympner, K.-D., P. K. Klose and R. B. Pelka. 1987. Natura Med. 2: 78-84.
Uhlmann, W.-J. 1958. Medizinische 2: 81-4.
Unruh, V. 1915. Nat. Eclec. Med. Assn. Q. 7: 63.
Verelis, C. and H. Becker. 1977. Planta Med. 31: 288.
Verelis, C. 1978. Dissertation, University of Heidelberg.
Viehmann, P. 1978. Erhahrungsheilkunde 27: 353.
Voaden, D.J. and M. Jacobson. 1972. J. Med. Chem. 15: 619.
Vogel, G. et al. 1968. Arzn.-Forsch. 18: 426.
Vogel V. 1977. American Indian Medicine, Norman, Oklahoma. University of Oklahoma Press.
Volz, G. 1957. Ther. Ggw. 96: 312.
Vömel, T. 1985. Arzn.-Forsch. 35: 1437.
Vorberg, G. and B. Schneider. 1989. Ärztl. Fortschr. 36: 3.
Wacker, A., et al. 1973. Arzneim.-Forsch. 23: 119.
Wacker, A. and A. Hilbig. 1978. Planta Med. 33: 89.
Wagner, H., H. Stuppner, J. Puhlmann, B. Brümmer, K. Deppe, and M.A. Zenk. 1989. Z. Phytother. 10: 35-8.
Wagner, H., A. Proksch, et al. 1981. Z. für Angewandte Phytotherapie 2: 166.
Wagner, H and A. Proksch. 1985a. In: Economic and Medicinal Plant Research. Orlando: Academic Press, p. 113.
Wagner, H. and K. Jurcic. 1991. Arzn.-Forsch. 41: 1072-6.
Webster, H. T. 1891. Massachusetts Medical J. 11: 344.
Wedel, W. 1936. Bur. Am. Ethn. Bull. 112: 59.
Weissbach, L., et al. 1977. Therapiewoche 27: 6009.
Wember, S. 1953. Landarzt. 29: 621.
Wood -. 1925. Propagateur de l'Homeopathie.
Woods, E. L. 1930. Am. J. Pharm. 102: 611-630.
Zimmermann, O. 1969. Hippokrates 40: 233.
Some references mentioned in the text pertain to material on the chemistry and pharmacology of Echinacea originally included in this piece. Space restrictions prevented inclusion here, but the material is presented in a supplementary publication, "Echinacea :Chemistry and Pharmacology."
Article copyright American Botanical Council.
~~~~~~~~
By Christopher Hobbs
|
|
|
|
|
|
|