Issue: 39 Page: 46
Chemistry of Kava.
by Mark Blumenthal, Yadhu N. Singh
HerbalGram. 1997; 39:46 American Botanical Council
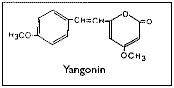
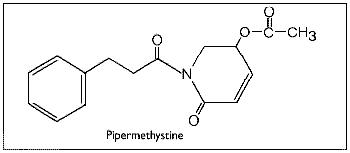
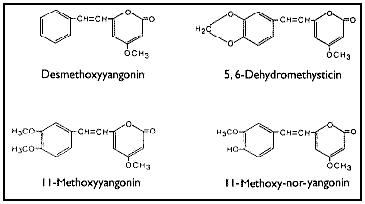
The reported pharmacological effects of kava drinking have prompted numerous chemical investigations over the past 130 years in the search for the biologically active constituents. These investigations have resulted in the isolation of two series of closely related compounds which are either substituted alpha-pyrones or substituted (5,6)-dihydro-alpha-pyrones, and some other miscellaneous compounds.Gobley(126) and Cuzent(127) almost simultaneously reported the isolation of the first of these compounds, now called methysticin, (previously known as kavakin, kawakin, kavatin, and kanakin). In 1874, Nölting and Kopp(128) reported a crystalline material whose isolation was later repeated by Lewin(96) and who named this compound yangonin. The isolation of dihydromethysticin (DHM) was achieved by Winzheimer in 1908.(129) Lewin's work in 1886 was followed by the most extensive chemical investigation ever undertaken on kava with the publication of 14 papers by Borsche and his co-workers during 1914-33. This work covered two new constituents, kawain and dihydrokawain (DHK) and their structural elucidation together with those of the three known components, methysticin, yangonin, and dihydromethysticin (DHM). However, Borsche and Bodenstein(130) incorrectly formulated yangonin as the 2-methoxy-4-pyrone structure. It was not until 1950 that the error was corrected by Macierewicz,(131) and independently confirmed.(132) It was finally unambiguously synthesized in 1960.(133) Four new compounds were added to the alpha-pyrone series: 5, 6-dehydromethysticin, II-methoxyyangonin and II-methoxynoryangonin(134), and desmethoxy-yangonin(135), together with the isolation of two pigment materials, flavokawin A and fiavokawin B. These pigments have been established by synthesis to be substituted chalcones.(136) According to Shulgin4 these otherwise biologically inactive pigments might explain the skin discoloration observed with chronic use of kava extracts. Depending on the separation procedures employed, early attempts to establish the relative amounts of the components present in kava have yielded uncertain results. Young et al.(137) used spectrophotometric analytical techniques while Klohs et al.(135) assayed the fractions from column chromatographic separations. Based on the available data,(136,137,138,139,140) Shulgin(4) has classified the proportion of each component present as being major (DHK, kawain and methysticin), minor (5, 6-dehydromethysticin, desmethoxyyangonin, DHM, flavokawin A, and yangonin), or trace (flavokawin B, II-methoxynoryangonin, and II-methoxyyangonin). Renewed interest in kava has prompted chemists to develop more rapid and reproducible detection techniques for the active kava components and their metabolites. Duve and co-workers(141,142,143,144) have used a gas-liquid chromatographic technique to identify the major active constituents, to assay the effectiveness of various extraction procedures during beverage preparation, and to determine the stability of the constituents in long-term storage of dried powdered roots and basal stems of the plant. On the other hand, Duffield and his group employed methane chemical ionization gas chromatography/mass spectroscopy in their studies by which they were able to confirm earlier studies and identify human urinary metabolites and previously unknown trace compounds of kava.(144-147) Despite earlier claims(129,149,150) that alkaloids were present in the roots, all attempts at their isolation were unsuccessful until 1971 when two amides in trace amounts were reported.(151) Recently, the isolation and structural determination of a novel alkaloid, pipermethystine, a major constituent of the leaves, has been achieved by Smith.(152) It is also present in small amounts in stems and roots of the plant, but due to its instability it had not previously been reported. In an effort to assign biological activity to one or the other portion of the molecule, several structural modifications of the active constituents have been carried out. Pharmacological assays of the analogues in various test systems suggest certain structure-activity relationships.(153) For more detailed accounts of the chemistry and structure-activity relationships,the reader should consult reviews by Keller and Klohs(3) and Shulgin(4) and the more recent papers of Duve's and Duffield's groups(140-147). 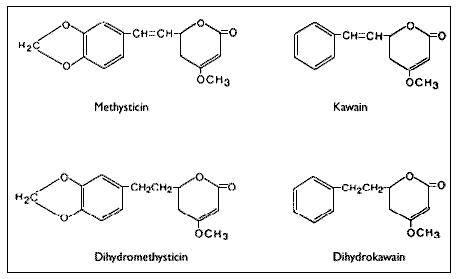
 Article copyright American Botanical Council.
Article copyright American Botanical Council. ~~~~~~~~
By Yadhu N. Singh and Mark Blumenthal
|