Introduction
Interest is growing in Germany about preparations made from the secondary tubers of the traditional African herb, devil’s claw (Harpagophytum procumbens), based on several recent clinical studies showing reduction of pain sensation and improved mobility within a few weeks of treatment. Also, in these studies patients were able to reduce dosages of standard antirheumatic drugs. Pharmacological studies on devil’s claw support analgesic and antiinflammatory actions. Extracts and drugs of the secondary tubers of devil’s claw are approved in monographs published by the German Commission E as well as by the European Scientific Cooperative on Phytotherapy (ESCOP). They appear to be safe and effective herbal remedies for the treatment of degenerative painful rheumatism, arthrosis (osteoarthritis) and tendonitis, often as an adjuvant therapy with conventional pharmaceutical drugs.
Botany and Nomenclature
Devil’s claw (Harpagophytum procumbens DC, Pedaliaceae) is found only in southern Africa.1 The natural habitats are Kalahari savannas and deciduous forests in Namibia and parts of the adjacent Republic of South Africa, Botswana, Angola, and Zimbabwe. The plant belongs to the same botanical family (Pedaliaceae) as sesame (Sesamum indicum).
Devil’s claw is a perennial herb. It has several prostrate annual stems from a succulent taproot, with additional tubers on lateral roots. At the beginning of the rainy season, the larger nodular roots produce flat-lying shoots. To survive the dry period, the plant forms water-storing secondary root tubers that branch off horizontally from the primary taproot. It produces large, hook-like fruit with rows of curved arms bearing recurved spines. Fruits may be up to 15 cm in diameter.1,2
The common name derives from the translation of the Namibian farmers’ German name, Teufelskralle, meaning devil’s claw.3,4 Other names are grapple plant and woolspider. The Harpago in the genus name translates to hook, a grappling hook or a drag, obviously based on the fearsome-looking fruits that can cripple a larger animal by becoming jammed in the foot or the hoof. In an animal’s mouth, it may firmly hook itself to the jaw. In this case, the animals cannot get rid of the obstruction and some have been known to starve to death. The hooked fruit may also become entangled in wool, mane, tail, or hair, where it remains with great tenacity.5
Harvesting
The medicinal material consists of the cut and dried secondary root tubers of the plant. The primary vertical root contains the same constituents but at lower levels than the secondary roots. These tubers are obtained by wild collection and by harvesting cultivated plant material on farms planted for continuous production for the medicinal market.6,7 Devil’s claw is grown and collected only in natural habitats in Southern Africa. Cultivation in other environments seems impossible.6,8
To harvest, the soil is shoveled, by hand, away from the stem to reveal the primary roots. From these, thin side roots branch off, at the end of which secondary storage roots (the tubers) might be found. These are collected, washed, sliced, and dried in the sun. To ensure continuous harvest in the next growing cycle, the holes are refilled with soil.7
History
A German soldier, Mehnert, introduced devil’s claw in Europe as an herbal tea in the mid-1900s.8,9 He discovered this herb by an intensive study of the local native medicine of the Bushman, Hottentot, and Bantu in Namibia.
The natives prized the tuber of devil’s claw as a bitter-tasting medicine, especially for stomach complaints (dyspepsia). Further, an infusion was recommended for the relief of all fevers, for blood diseases, and as an anti-
inflammatory and analgesic agent. It was administered to pregnant women to relieve postpartum pain. Ointments are applied to sprains, sores, ulcers, and boils.2,5 In general, Africans have used devil’s claw tubers for centuries, if not millennia.
Modern studies document the effects of devil’s claw and are still ongoing. The German Commision E and ESCOP monographs allow the use of devil’s claw in arthrosis (osteoarthritis) and tendonitis.10,11 The recommended use in dyspepsia is valid only when administered in bitter-tasting preparations.
Phytochemistry
The cut and dried secondary root tubers of devil’s claw yield a variety of compounds, mainly iridoid glycosides (up to 3 percent), which are considered pharmacologically active. The fraction of iridoid glycosides consists of harpagoside, procumbide, harpagid, and 8-para-coumaroyl-harpagid. Harpagoside is the primary iridoid glycosides.6,12-15 Iridoids were not considered previously as a particularly important pharmacologically active class of compounds. More recently, extensive investigations into their biological activity in general and their potential pharmacological activity in particular have revealed that iridoids exhibit a wide range of bioactivity. They are now known to be present in a number of folk medicines used as bitter tonics, sedatives, febrifuges, cough medicines, remedies for wounds and skin disorders, and as hypotensives.16
Additional constituents with probable activity are glycosides of the flavonoids, kaempferol and luteolin, chlorogenic acid and cinnamic acid, the phenylethanoid acteoside, quinone, harpagoquinone, triterpenes like ursolic and oleanic acid and derivatives.4,9 Some papers reported on a direct inhibition of cyclooxygenase-2 (COX-2) catalyzed prostaglandin biosynthesis or COX-2 activity by the flavonoid kaempferol as well as ursolic and oleanic acid.17-19
Pharmacology
The first scientist to study the pharmacological effects of devil’s claw was Zorn at the University of Jena, Germany, more than 40 years ago.20 Based on his positive findings, further experimental and clinical studies followed, contributing to the therapeutic profile of this phytomedicine.
The most important of the Commission E recommended uses are its antiinflammatory and analgesic effects. Research also suggests antiarrhythmic and hypotensive effects; however, these actions have not stimulated clinical interest in devil’s claw. In all these studies, the dosages of the extracts were about 20—1,200 mg drug material per kilogram of body weight.
Some reports of antiinflammatory activity of devil’s claw in animal experiments conflict. Some studies show a strong effect, while others fail to show positive effects. Antiinflammatory effects have been demonstrated more convincingly in recurrent conditions, rather than in acute.8 This is consistent with the recommended use in chronic rheumatic diseases. In conclusion, the experimental findings may explain the underlying analgesic and antiinflammatory effects for both the whole extracts and isolated constituents of devil’s claw. Conflicting results might be explained by different extract qualities and by different methodological designs. In general, most positive results were achieved following oral administration of aqueous extracts compared to alcoholic extracts or isolated constituents or parental application. (For summaries of the pharmacological and pharmacokinetic literature, see the related story, on page 52.)
Clinical Data and Modern Use
In Europe the clinical use of devil’s claw is restricted to applications in rheumatism and dyspepsia. However, its use as a dyspeptic aid was limited to infusions (herbal teas), available in the first decades of marketing of devil’s claw teas in Europe. The dyspeptic action may be due to the strong and intensive stimulatory bitterness of the dried tuber. It is not known whether drug material in the processed state (e.g., extracts) exerts a comparable antidyspeptic effect. In clinical studies testing for effects of solid preparations (e.g., capsules or tablets) of devil’s claw for rheumatic complaints, neither physicians nor patients made comments to support an antidyspeptic effect.
Many studies have assessed the efficacy of devil’s claw in the relief of arthrosic (osteoporotic) and arthritic conditions.8,9,21 The studies support the approved indications in the positive monographs produced by ESCOP and the German Commission E: painful arthrosis and tendonitis10 and for supportive or adjuvant treatment of degenerative rheumatism ("degenerative disorders of the locomotor system").11 The use of devil’s claw for degenerative rheumatism today may be due to the positive data for this indication from clinical and pharmacological studies. The pharmacological data support an adjuvant effect on arthritis; however, there are only very limited data available.
Devil’s claw is used primarily to improve pain, mobility and motility of patients with arthrosic and arthritic conditions. In addition, new studies showing successful use of devil’s claw have been published in the last few years (see Table 1).
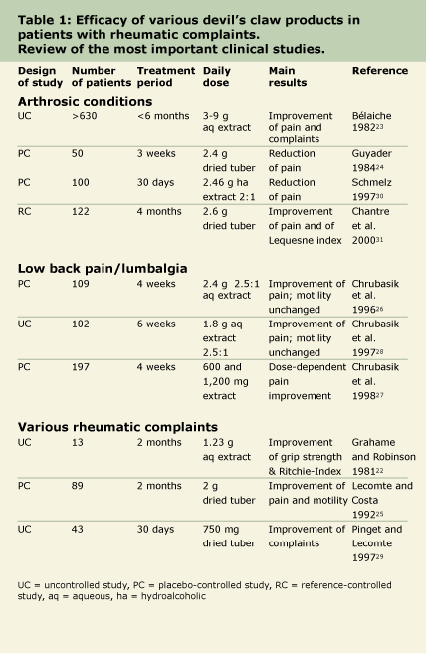
In one study, an insignificant improvement of grip strength and Ritchie-index (an index for the flexibility of the trunk) were reported in 13 patients, suffering mainly from seropositive arthritis, after a six-week treatment of 1,230 mg per day of devil’s claw extract (unspecified concentration of aqueous dry extract, Salus, Germany).22
In a large uncontrolled study, 630 patients suffering from arthrosis of hip, knee, fingers, and spine were treated for six months with devil’s claw aqueous dry extract (standardized to 2.5 percent of iridoid glycosides) at a daily dosage of 3 to 9 g.23 Improvement of pain sensation and other complaints was demonstrated in 42 percent to 85 percent of the patients, according to localization of arthrosis. No side effects other than mild gastrointestinal disturbances were reported, even at the highest dosage level.
In a double-blind study, 50 patients with arthrosis received three doses totalling 2,400 mg per day of devil’s claw (each dose was two capsules of 400 mg cryoground dried root material, standardized to 1.5 percent iridoid glycosides) up to three times per week for a three-week period.24 Severity of pain was assessed 10 days after treatment completion. Compared with placebo, the extract significantly decreased the severity of the patients’ pain.
In a double-blind study25 of 89 patients with rheumatic articulation joint pain, the efficacy and tolerance of a daily dose of 2,000 mg of powdered devil’s claw (three times daily, 2 capsules, each 335 mg of powdered cryoground drug material, standardized to 3.0 percent of iridoid glycosides; Arkopharma, France) for two months was assessed. The clinical parameters measured on days 0, 30, and 60, severity of Visual Analog Scale (VAS) pain and joint mobility determined by finger-floor distance, revealed a significant drop in the intensity of pain and a significant increase in mobility in the treatment group. Neither side effects nor changes in laboratory parameters were observed during the two-month study.
A four-week placebo-controlled double-blind study with a daily dosage of 2,400 mg of devil’s claw extract (three times, two tablets, 400 mg each 2.5:1 aqueous dry extract, Doloteffin®, Ardeypharm, Germany) tested patients with acute exacerbations of chronic low back pain. The outcome was measured by a validated low back pain index, and scales to measure pain sensation, back mobility, and overall patient mobility. Of the 118 original patients, 105 completed the study; nine in the treatment group and one in the placebo group were pain free at the end of treatment. There was a median improvement of the low back pain index of 20 percent compared to the initial value in the devil’s claw group compared to 8 percent of placebo. This trend was related to a significant decrease in the pain index. Only minor nonspecific adverse effects were reported.26
In a placebo-controlled double-blind study, 197 patients suffering from chronic local, as well as radiating, low back pain for at least six months were treated with 600 or 1,200 mg of devil’s claw extract (three tablets of 200 or 400 mg each, WS 1532, Schwabe, Germany; dosages corresponding to 50 or 100 mg harpagoside per day) for four weeks.27 The outcome was measured by the low back pain index as in the previous study by the same research team.26 Of the 182 patients who completed the study, the number of pain-free patients increased dose-dependently: 3, 6 and 10 patients of placebo, 200 and 400 mg harpagoside, respectively. Adverse events were not reported.
In a controlled study, 102 patients suffering from acute local low back pain for more than six months were treated with 1,800 mg devil’s claw extract (2.5:1 aqueous dry extract, Jucurba®, Strathmann, Germany) or with conventional treatment (a nonsteroidal anti-
inflammatory drug, NSAID) for six weeks.28 Again, the outcome was measured by the low back pain index. The percentage of pain-free patients after four and six weeks of treatment was similar in both groups (devil’s claw, 32 percent and 29 percent; NSAID group, 23 percent and 45 percent, respectively). Six weeks after initiation of treatment, the low back pain index improved in both groups about 20 percent. The relative change of the single components measured – pain, mobility, and physical impairment – did not differ between the groups. However, in both groups, the pain index decreased significantly from week 4 to 6 of treatment. Only minor adverse events were reported in the devil’s claw group, not necessitating discontinuation of treatment.
Forty-three patients with osteoarthritis and rheumatoid arthritis were enrolled in an uncontrolled study with a daily dosage of 750 mg powdered secondary tubers of devil’s claw (Arkogélule d’Harpagophytum, Arkopharma, France) for a course of 30 days.29 At the end of treatment patients reported significant improvement of symptoms, mobility, and morning stiffness. Adverse events were not reported.
In a double-blind, placebo-controlled study, the analgesic effect of a devil’s claw extract (2,460 mg of hydroalcoholic dry extract daily, 2:1, 40 percent ethanol; Pagosid®, Dr. Duenner, Switzerland) was investigated in 100 patients with osteoarthritis, chronic low back pain and myalgia.30 Following 30 days of treatment, only six patients reported a strong, and one patient, a medium pain sensation, compared to 32 and nine in the placebo group, respectively. Only one patient of the treatment group reported diarrhea as an adverse event.
In a recent double-blind, randomized, multicenter clinical study, the action of powdered cryoground devil’s claw tuber, about 2,600 mg daily (Harpadol®, Arkopharma, France), was studied for four months in 122 patients suffering from osteoarthritis of knees and hips.31 The action was compared with that of 100 mg daily diacerhein, an anthraquinone derivative producing rhein (the actual active component); diacerhein is approved as a conventional osteoarthritis treatment in France and Italy. Spontaneous pain as evaluated by visual analog scale showed a significant improvement during the course of the study: about 50 percent in the devil’s claw group and about 58 percent in the control group. Similarly, there was a progressive and significant reduction in the Lequesne functional index (an international index for the evaluation of severity of osteoarthritis). Patients taking devil’s claw used significantly fewer NSAIDs and analgesic drugs and the frequency of adverse events was significantly lower in the devil’s claw group.
No significant effects on the mediators of acute inflammation (prostaglandin E2, thromboxane B2, 6-ketoprostaglandin F1a, and leukotriene B4) was measured in 25 healthy volunteers after a three-week daily intake of 2 g of powdered devil’s claw containing 3 percent iridoid glycosides.32 The subjects served as their own control and were also compared with a separate control group. However, this study was not consistent with dosage and duration recommendations as noted in monographs published by ESCOP (1—3 g of root in decoction three times daily or 1—3 g dried root or equivalent preparations daily for 2—3 months)10 and the German Commission E, i.e., 4,500 mg daily (no duration noted).11 That is, the study’s negative outcome might be attributed to the fact that the dosage was about 33 to 60 percent lower than the upper range noted by ESCOP and Commission E, respectively, and the duration was significantly shorter than that recommended by ESCOP.
Therapeutic Safety
Devil’s claw preparations from dried tubers, drug, or extract, appear to be well tolerated in all the studies described above. In only a few cases, mild gastrointestinal complaints occurred. There were no reports of serious or major adverse effects. Use of devil’s claw with patients who have gastric and duodenal ulcers is contraindicated because bitter-tasting preparations are believed to stimulate gastric acid secretion. There are no reports of negative interactions with conventional drugs usually prescribed for rheumatic or arthrosic conditions.
Conclusions
Dried tuber and extracts of secondary root of devil’s claw are interesting therapeutic remedies for the adjuvant treatment of painful arthrosis (osteoarthritis) and tendonitis. As has been shown in numerous clinical studies in patients with rheumatoid arthritis, osteoarthritis, and lower back pain, devil’s claw preparations reduce pain sensation and improve mobility and motility of patients, and therefore increase quality of life within the first weeks of treatment. Further, the dosage of conventional antirheumatic drugs might be reduced.
The ESCOP and Commission E monographs recommend a daily oral dose of 1,000—4,500 mg of devil’s claw crude drug (dried tuber) or corresponding extracts. However, based on the results of recent studies, dosage should be as much as 4,500 mg of dried tuber or equivalent extracts for a noticeable effect in about four weeks of treatment.
Tankred Wegener is a consultant to the phytomedicine industry in Rheda-Wiedenbrueck, Germany, email: <tankred.wegener@t-online.de>, website <www.consulting-hmp.de>.
REFERENCES
1. Ihlenfeldt, H.D. Flora Zambesiaca. 125. Pedaliaceae 1988;8:86-118.
2. Iwu MM. Handbook of African Medicinal Plants. Boca Raton, FL: CRC Press; 1993.
3. Tyler VE. The Honest Herbal, 3rd ed. New York: Pharmaceutical Products Press; 1993.
4. Wenzel P, Wegener T. Teufelskralle–ein pflanzliches Antirheumatikum. Dtsch Apoth Ztg 1996;135:1131-44.
5. Watt M, Breyer-Brandwijk G. The Medicinal and Poisonous Plants of Southern and Eastern Africa. 2nd ed. London: Livingstone; 1962.
6. Schmidt M, Eich J, Kreimeyer J, Betti G. Anbau der Teufelskralle. Dtsch Apoth Ztg 1998;138:4540-9.
7. Schneider E. Sustainable use in semi-wild populations of Harpagophytum procumbens in Namibia. Medicinal Plant Conservation 1997;4:7-9.
8. Wegener T. Die Teufelskralle (Harpagophytum procumbens DC) in der Therapie rheumatischer Erkrankungen. Ztschr Phytother 1998;19:284-94.
9. Wegener T. Therapie degenerativer Erkrankungen des Bewegungsapparates mit südafrikanischer Teufelskralle (Harpagophytum procumbens DC). Wien Med Wschr 1999;8(10):254-257.
10. ESCOP. Monography Harpagophyti radix. In: ESCOP (eds.). Monographs on the Medicinal Uses of Plant Drugs, Vol. 2. Centre of Complementary Health Studies, University of Exeter, Exeter; 1996:1-7.
11. Blumenthal M, Busse WR, Goldberg A, Hall T, Riggins CW, Rister RS (eds.). Klein S, Rister RS (trans.). The Complete German Commission E Monographs: Therapeutic Guide to Herbal Medicines. Austin: American Botanical Council, Boston: Integrative Medicine Communications; 1998. (Monograph Harpagophyti radix, Südafrikanische Teufelskrallenwurzel. Banz No. 43 of 2.3.1989, corrected in Banz No. 164 of 1.9.1990.)
12. Czygan FC, Krueger A, Schier W, Volk OH. Pharmazeutisch-biologische Untersuchung der Gattung Harpagophytum (Burch.) DC ex Meissn; 1. Mitteilung: Phytochemische Standardisierung von Tubera Harpagophyti. Dtsch Apoth Ztg 1977;117:1431-4.
13. Eich J, Schmidt M, Betti G. HPLC analysis of iridoid compounds of Harpagophytum taxa: Quality control of pharmaceutical drug material. Pharm Pharmacol Lett 1998;4:75-8.
14. Guillerault L, Ollivier E, Elias R, Balansard G. Determination of harpagide, 8-para-coumaroyl harpagide, and harpagoside by high performance liquid chromatography in Harpagophytum procumbens drugs and in a commercial extract. J Liquid Chrom 1994;17:2951-60.
15. Sticher O, Meier B. Quantitative Bestimmung von Harpagosid in Wurzeln von Harpagophytum procumbens mit Hochleistungsflüssigkeitschromatographie (HPLC). Dtsch Apoth Ztg 1980;120:1592-4.
16. Ghisalberti EL. Biological and pharmacological activity of naturally occuring iridoids and secoiridoids. Phytomedicine 1998;5:147-63.
17. Liang YC, Haung YT, Tsai SH, Lin-Shiau SY, Chen CF, Lin JK. Suppression of inducible cyclooxygenase and inducible nitric oxide synthase by apigenin and related flavonoids in
mouse macrophages. Carcinogenesis 1999;20:1945-52.
18. Mutoh M, Takahashi M, Fukuda K, Matsushima-Hibiya Y, Mutoh H, Sugimura T, Wakabayashi K. Supression of cyclooxygenase-2 promoter-dependent transcriptional activity in colon cancer cells by chemopreventive agents with a resorcin-type structure. Carcinogenesis 2000;21:963-9.
19. Ringbom T, Segura L, Noreen Y, Perera P, Bohlin L. Ursolic acid from Plantago major, a selective inhibitor of cyclooxygenase-2 catalyzed prostaglandin biosynthesis. J Nat Prod 1998;61:1212-5.
20. Zorn B. Ueber die antiarthritische Wirkung der Harpagophytum-Wurzel. Z Rheumaforsch 1985;17:134-8.
21. Schulz V, Haensel R, Tyler VE. Rational Phytotherapy, A physician´s guide to herbal medicine, 3rd ed. Berlin: Springer; 1998.
22. Grahame R, Robinson BV. Devil’s Claw (Harpagophytum procumbens): pharmacological and clinical studies. Ann Rheum Dis 1981;49:632.
23. Bélaiche P. Étude clinique de 630 cas d´arthrose traités par le nébulisat aqueux d´Harpagophytum procumbens (Radix). Phytothérapy 1982;1:22-8.
24. Guyader M. Les plantes antirhumatismales. Étude historique et pharmacologique, et étude clinique du nébulisat d’Harpagophytum procumbens DC chez 50 patients arthrosiques suivis in service hospitalier. [Dissertation]. Université Pierre et Marie Curie, Paris, 1984
25. Lecomte A, Costa JP. Harpagophytum dans l´arthrose. Etude en double insu contre placebo. 37°2 Le Magazine 1992;27-30.
26. Chrubasik S, Zimpfer C, Schuett U, Ziegler R. Effectiveness of Harpagophytum procumbens in treatment of acute low back pain. Phytomedicine 1996;3:1-10.
27. Chrubasik S, Junck H, Conradt C, Zappe H, Chrubasik J. Effectiveness of oral Harpagophytum extract WS 1531 in treating low back pain. Arthr Rheum 1998;41(suppl. 9):S261.
28. Chrubasik S, Schmidt A, Junck H, Pfisterer M. Wirksamkeit und Wirtschaftlichkeit von Teufelskrallenwurzelextrakt bei Rückenschmerzen: Erste Ergebnisse einer therapeutischen Kohortenstudie. Forsch. Komplementärmed 1997c;4:332-6.
29. Pinget M, Lecomte A. Die Wirkung der "Harpagophytum Arkocaps" bei degenerativem Rheuma. Naturheilpraxis 1997;50:267-9.
30. Schmelz H, Haemmerle HD, Springorum HW. Analgetische Wirksamkeit eines Teufels-krallenwurzel-Extraktes bei verschiedenen chronisch-degen-erativen Gelenkerkrankungen. In: Chrubasik S, Wink M (eds.): Rheumatherapie mit Phytopharmaka. Stuttgart: Hippokrates; 1997:86-9.
31. Chantre P, Cappelaere A, Leblan D, Guedon D, Vandermander J, Fournie. Efficacy and tolerance of Harpagophytum procumbens versus diacerhein in treatment of osteoarthritis. Phytomedicine 2000;7:177-83.
32. Moussard C, Alber D, Toubin MM, Thevenon N, Henry JC. A drug used in traditional medicine, Harpagophytum procumbens: No evidence for NSAID-like effect on whole blood eicosanoid production in human. Prostaglandins Leukotriens and Essential Fatty Acids 1992;46:283-6.
33. Eichler O, Koch C. Über die antiphlogistische, analgetische und spasmolytische Wirksamkeit von Harpagophytum procumbens. Arzneimittel-Forsch/Drug Res 1970;20:107-9.
34. Erdoes A, Fontaine R, Friehe H, Durand R, Poeppinghaus T. Beitrag zur Pharmakologie und Toxikologie verschiedener Extrakte, sowie des Harpagosids aus Harpagophytum procumbens DC. Planta Med 1978;34:97-108.
35. Whitehouse LW, Znamirowska M, Paul CP. Devil’s Claw (Harpago-phytum procumbens): no evidence for anti-inflammatory activity in the treatment of arthritic disease. Can Med Assoc J 1983;129:249-51.
36. Manez S, Alcaraz MJ, Payá M, Rios JL, Hancke JL: Selected extracts from medicinal plants as anti-inflammatory agents. Planta Med 1990;56:656.
37. Jadot G, Lecomte A. Activité anti-inflammatoire d’Harpagophytum procumbens DC; Lyon Méditerranée Médical Médicin du Sud-Est 1992;28:833-5.
38. Lanhers MC, Fleurentin J, Mortier F, Vinche A, Younos C. Anti-Inflammatory and Analgesic Effects of an Aqueous Extract of Harpagophytum procumbens. Planta Med 1992;58:117-23.
39. Soulimani R, Younos C, Mortier F, Derrieu C. The role of stomachial digestion on the pharmacological activity of plant extracts, using as an example extracts of Harpagophytum procumbens. Can. J Physiol Pharmacol 1994;72:1532-6.
40. Baghdikian B, Guiraud-Dauriac H, Ollivier E, N´Guyeb A, Dumenil G, Balansard G. Formation of nitrogen-containing metabolites from the main iridoids of Harpagophytum procumbens and H. zeyheri by human intestinal bacteria. Planta Med 1999;65:164-5.
41. Fleurentin J, Mortier F. Entzuendungshemmende und analgetische Wirkungen von Harpagophytum procumbens und H. zeyheri. In: Chrubasik S, Wink M (eds.): Rheumatherapie mit Phytopharmaka. Stuttgart: Hippokrates: 1997:68-76.
42. Costa de Pasquale R, Busà G, Circosta C, Lauk L, Ragusa S, Ficarra P, Occhiuto F. A drug used in traditional medicine: Harpagophytum procumbens DC. J Ethnopharmacol 1985;13:193-9.
43. Circosta C, Occhiuto F, Ragusa S, Trovato A, Tumino G, Briguglio F, de Pasquale A. A drug used in traditional medicine: Harpagophytum procumbens DC—II. Cardiovascular activity. J Ethnopharmacol 1984;11:259-274.
44. Chrubasik S. Biopharmazeutische Qualität und klinische Wirksamkeit von Zubereitungen aus Harpago-phytumextrakt. In: Chrubasik S, Wink M (eds.). Rheumatherapie mit Phytopharmaka. Stuttgart: Hippokrates; 1997:77-85.
45. Chrubasik S. Teufelskral-lenwurzelextrakt. Klinisch geprüfte Wirksamkeit bei akuten Rückenschmerzen. Der Allgemeinarzt 1997;19:564-8.
46. Loew D, Schuster O, Moellerfeld J. Stabilität und biopharmazeutische Qualität. Voraussetzung für Bioverfügbarkeit und Wirksamkeit von Harpagophytum procumbens. In: Loew D, Rietbrock N (eds.). Phytopharmaka II: Forschung und klinische Anwendung. Darmstadt: Steinkopff; 1996:83-94.
47. Baghdikian B, Lanhers MC, Fleurentin J, Ollivier E, Maillard C, Balansard G, Mortier F. An analytical study, anti-inflammatory and analgesic effects of Harpagophytum procumbens and Harpagophytum zeyheri. Planta Med 1997;63:171-6.
48. Tippler B, Syrovets T, Loew D, Simmet T. Harpagophytum procumbens: Wirkung von Extrakten auf die Eicosanoidbiosynthese in Ionophor A23187-stimuliertem menschlichem Vollblut. In: Loew D, Rietbrock N (eds.). Phytopharmaka II. Forschung und klinische Anwendung. Darmstadt: Steinkopff; 1996:95-100.
Vanhaelen M. La biochimie et l´activité de Harpagophytum procumbens et de Glycorrhiza glabra. Toxicité de Symphytum consolida. J Pharm Belg 1986;41:172-82.
Sidebar: