Issue: 61 Page: 69-70
Kava Stakeholders Plan Regulatory Review and Market Return
by Joerg Gruenwald
HerbalGram. 2004; 61:69-70 American Botanical Council
The roads of the Fiji islands of Taveuni are empty these days.
A thriving economy based on the export of the traditional Pacific crop kava (Piper
methysticum G. Forst., Piperaceae) collapsed in 2001 due to allegations in
Europe that the plant extract caused liver toxicity.1 This shocked
native island people as their ancestors had been drinking kava extracts for thousands
of years for ceremonial and social purposes without such apparent effect. An explanation
of this surprising turn of events was suggested at the recent meeting in Brussels,
Belgium, of Pacific and European kava stakeholders.
The first-ever European-Pacific Kava Stakeholders meeting was
held in Brussels on August 25 and 26, 2003, organized by the European Centre for
the Development of Enterprise (CDE) and PRO€INVEST, both based in Brussels.
The main objective of the meeting was to explore ways to re-establish the kava
trade between the European Union (EU) member states and South Pacific countries.
A major focus of discussion was the main findings of the study entitled "In-Depth
Investigation of EU Member States Market Restrictions on Kava Products" (Phytopharm
Report) presented by Phytopharm Consulting at the end of March 2003. The entire
report can be downloaded from <www.analyze-realize.com/Publications/Kava.en.html>.2
The report was commissioned by the EU CDE, on behalf of some
kava-producing countries in the South Pacific and the Pacific Island Forum Secretariat
(PIFS), to critically evaluate whether or not the restrictions placed on kava
by some European health authorities are justified. It included an independent
expert report on the pharmacological and clinical documentation of kava (Phytopharm
Report part IIA and B) and a detailed case analysis of all reported cases of hepatotoxic
events (Phytopharm Report part IIA Annex 1). The findings presented in the Phytopharm
Report clearly speak for the safety and efficacy of kava in the symptomatic treatment
of anxiety and stress and, in most aspects, strongly supported the scientists’
charges that the kava ban was not justified based on the available scientific
and medical evidence. Furthermore, the report criticized the German health authority
(Bundesinstitut für Arzneimittel und Medizinprodukte or BfArM, the
Federal Institute for Drugs and Medical Devices) for having ignored and misinterpreted
important scientific data in its evaluation and for creating an obviously distorted
image for kava.
The meeting brought together key participants from the Pacific
Region and Europe, representing a cross section of stakeholders, namely European
manufacturers, regulatory agencies, kava exporters, scientists and experts, and
organizations such as the CDE, PRO€INVEST, PIFS, Commonwealth Secretariat
(COMSEC), European Commission (EC), the World Health Organization (WHO), and the
Technical Centre for Agricultural and Rural Cooperation ACP-EU (CTA). The meeting
also reviewed alternative strategies that might be used to reintroduce kava into
European markets, established a detailed action plan, and identified the possible
roles and contribution of the different organizations and representatives in achieving
these goals.
Kava Strategy: The Way Forward
The stakeholders acknowledged the significant economic,
social and cultural damage done by the market recalls, restrictions, and bans.
They noted that the negative publicity from the numerous kava bans, alerts, and
market recalls had led to an adverse economic impact for the South Pacific kava
industry predominantly located in Fiji, Samoa, Tonga, and Vanuatu. Since 2001
loss of local export earnings in the South Pacific was more than US$200 million,
and many thousands of jobs in both the South Pacific and Europe. The ban especially
affected the incomes of rural farmers and processors as well as foreign exchange,
as exports are seriously adversely affected. The meeting further noted the loss
of business for the European importers.
The participants also endorsed the findings of the Phytopharm
Report, and agreed to use its scientific evidence, which clearly indicate that
the bans, sales restrictions, and market recalls by the regulators in respective
EU countries were unjustified (see Table 1). Such bans and restrictions also ignored
the huge body of positive evidence on efficacy and safety of kava.
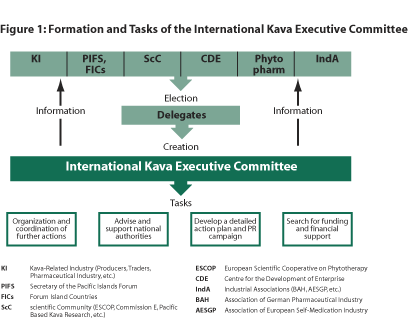
The stakeholders agreed to establish an International Kava
Executive Committee (IKEC) comprising representatives of the stakeholders and
organizations to address immediate and future issues relating to the management
of kava as proposed in the strategy of the Phytopharm Report (see Figure 1).
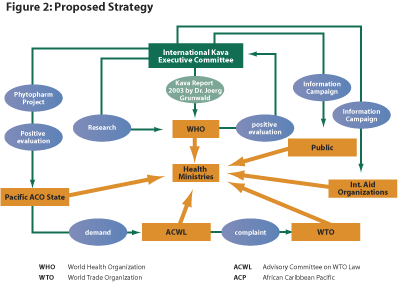
Those present also adopted the proposed strategy in the Phytopharm
Report presented in Figure 2 and the summarized and amended version of this strategy
performed by the representative of the Pacific Islands Forum Secretariat.
As a matter of immediate priority, the stakeholders agreed
to undertake the following actions:
1. Establish an International Kava Executive Committee (IKEC)
of EU and Pacific members to implement the coordinated strategy for re-establishing
the kava market in Europe, consisting of the following:
Pacific Islands Stakeholders
• Four Pacific Island countries:
— Josateki Nawalowalo (Fiji Kava Council);
— Eddi Wilson (Samoa Association of Manufacturers and
Exporters);
— Toimoana Takataka (Tonga Kava Council);
— Frank King (Vanuatu Kava Exporters Association);
• Pacific Islands Forum Secretariat.
European Stakeholders
• Barbara Steinhoff (Bundesverband der Arzneimittelhersteller
or German Medicines Manufacturers’ Association);
• Mathias Schmidt (Representative of the Kava Industry);
• Anthony Bush (European Federation of Health Product
Manufacturers Associations);
• Carlo Sessa (Associazione Italiana fra Coltivatori,
Raccoglitori, Trasformatori, Importatori, Esportatori, Grossisti e Rappresentanti
di Case Estere di Piante Medicinali e Aromatiche or Italian Medicinal and
Aromatic Plants Producer and Traders Association).
Executive Director
• Joerg Gruenwald (Phytopharm Consulting)
2. Request funding from CDE/PRO€INVEST for Phytopharm
to become the active coordinator of the activities in Europe.
3. Take immediate steps in addressing the following:
• Provide German and other relevant authorities with
the Phytopharm Report;
• Pacific members to request WHO for a re-evaluation
of safety and efficacy of kava by an independent expert commission;
• CDE/PRO€INVEST to support the proposed strategy
by providing technical assistance, and lobbying support to the IKEC.
4. Use the scientific evidence to dispel fears of harm from
kava and kava-based products.
5. Encourage a plan of action to strengthen the kava industry
in the Pacific with appropriate training programs and standards compliance.
6. Secure funding from International Donor Agencies to initiate
various activities.
7. Circulate the Phytopharm Report as widely as possible,
including those EU countries that have not banned kava, to use the report to prevent
any future bans.
Finally, the stakeholders expressed their sincere appreciation
to the CDE, PRO€INVEST, and PIFS for their effective roles in undertaking
the study and organization of the meeting. The meeting also acknowledged the ongoing
support of these organizations and others such as COMSEC, WHO, and the EU in addressing
the problems associated with kava and kava products.
Joerg Gruenwald is president of Phytopharm Consulting,
a specialized business consulting company for herbal medicine, dietary supplements,
and functional foods. He is author of the international reference work for botanical
medicines Physician Desk Reference for Herbal Medicines, co-editor of
The Complete German Commission E Monographs, and editor in chief of Advances
in Natural Therap. Dr. Gruenwald is the executive director of the International
Kava Executive Committee. He can be reached at Phytopharm Consulting, Waldseeweg
6, 13467 Berlin, Germany; +49-30-40008100, Fax: +49-30-40008500; e-mail: <jgruenwald@phytopharm.org>;
website: < www.analyze-realize.org>.
References:
1. Blumenthal M. Kava safety questioned due to case reports
of liver toxicity. HerbalGram 2002;55:26-32.
2. Gruenwald J, Mueller C, Skrabal J. In-Depth Investigation
of EU Member States Market Restrictions on Kava Products. Phytopharm Consulting,
2003. Accessed December 2, 2003 at URL: <www.analyze-realize.com/Publications/Kava.en.html>.
|