Issue: 62 Page: 33-48
Devil's Club (Oplopanax horridus): An Ethnobotanical Review
by Trevor C. Lantz, Kristina Swerhun, Nancy J. Turner
HerbalGram. 2004; 62:33-48 American Botanical Council
Devil’s Club (Oplopanax horridus): An Ethnobotanical Review
Devil’s club (Oplopanax horridus (Sm.) Torr. & A. Gray ex. Miq., Araliaceae)
is probably the most important spiritual and medicinal plant to most indigenous
peoples who live within its range. Different parts of this plant are used by over
38 linguistic groups for over 34 categories of physical ailment, as well as many
spiritual applications. Devil’s club [syn. Echinopanax horridus (Sm.) Decne.
& Planch, Fatsia horrida (Sm.) Benth. & Hook, Panax horridum Sm.;
Riconophyllum horridum Pall.] is a common deciduous understory shrub occurring
in moist, but well drained, forested ecosystems from coastal Alaska southward
to central Oregon and eastward to the southwestern Yukon, the Canadian Rockies,
northwestern Alberta, Montana, and Idaho. There are also several disjunct populations
near northern Lake Superior in Michigan and Ontario. The stems of this shrub are
upright to decumbent and can reach heights exceeding 6 meters (~20 feet). The
leaves are large (up to 35 cm across [~14 inches]) and maple-shaped.
 The
stems, petioles, and leaf veins of devil’s club are covered with a dense armor
of yellowish needle-like spines up to 2 cm (~0.5 inches) long, which can cause
severe skin irritation. The flowers are small and whitish, borne in terminal pyramidal
clusters, and ripen to shiny flattened, bright red berries. Devil’s club forms
large sprawling clones that expand laterally through the layering of decumbent
stems.1 The
stems, petioles, and leaf veins of devil’s club are covered with a dense armor
of yellowish needle-like spines up to 2 cm (~0.5 inches) long, which can cause
severe skin irritation. The flowers are small and whitish, borne in terminal pyramidal
clusters, and ripen to shiny flattened, bright red berries. Devil’s club forms
large sprawling clones that expand laterally through the layering of decumbent
stems.1
A member of the family Araliaceae (which also contains the
ginsengs), devil’s club is related to a number of widely known medicinals
including Asian ginseng (Panax ginseng
C.A. Meyer), American ginseng (P. quinquefolius L.),
eleuthero (Eleutherococcus senticosus Maxim., formerly called Siberian ginseng), and small
spikenard (Aralia nudicaulis L.,
or sarsaparilla). Devil’s club is often cited as the most significant plant,
both medicinally and spiritually, to the indigenous peoples within its range.2-5
The first ethnographic record of devil’s club use dates back to 1842, when
Eduardo Blaschke, the chief physician for the Russian American Company,
reported the use of devil’s club ash as a treatment for sores amongst the
Tlingit.6 Subsequently, devil’s club has received widespread
documentation for its medicinal, spiritual, and technological uses in
ethnographies, ethnobotanies, medical journals, and historical records from
within (as well as outside) its geographical range. In a 1982 review, Turner
reported more than 30 categories of medicinal, spiritual, and technological
uses by peoples of over 25 different indigenous linguistic groups of western
North America.4 Phytochemical research has revealed that this plant
has antifungal, antiviral, antibacterial, and antimycobacterial properties, and
these are undoubtedly related to its widespread use in traditional medicine.7-11
 Recent
commercial use of devil’s club seems to have developed in response primarily to
ethnographic records and the phytochemical research that these have inspired.
In most cases the use of devil’s club in the herbal and nutraceutical industries
parallels traditional uses described in ethnographic and ethnobotanical records.
Recently, however, devil’s club’s botanical relationship to the well-known medicinal
ginsengs (Panax ginseng, P. quinquefolius) has been used to increase its
commercial appeal. Thus, it is sometimes marketed under the misleading, and now
illegal in the United States, common names of “Alaskan ginseng,” “wild armored
Alaskan ginseng,”12 and “Pacific ginseng.”13 Such marketing
relies on purported phytochemical similarities between devil’s club and Panax
spp. Although largely unsubstantiated, presumably such claims are based on
the speculation that plants of the family Araliaceae may share similar chemical
constituents, a presumption that is not supported by current phytochemical research. Recent
commercial use of devil’s club seems to have developed in response primarily to
ethnographic records and the phytochemical research that these have inspired.
In most cases the use of devil’s club in the herbal and nutraceutical industries
parallels traditional uses described in ethnographic and ethnobotanical records.
Recently, however, devil’s club’s botanical relationship to the well-known medicinal
ginsengs (Panax ginseng, P. quinquefolius) has been used to increase its
commercial appeal. Thus, it is sometimes marketed under the misleading, and now
illegal in the United States, common names of “Alaskan ginseng,” “wild armored
Alaskan ginseng,”12 and “Pacific ginseng.”13 Such marketing
relies on purported phytochemical similarities between devil’s club and Panax
spp. Although largely unsubstantiated, presumably such claims are based on
the speculation that plants of the family Araliaceae may share similar chemical
constituents, a presumption that is not supported by current phytochemical research.
The prospect of increasing demand in the market has the
potential to increase the unregulated harvest of devil’s club. This is of
concern because this shrub is sensitive to over-harvesting.14 Since
devil’s club is extremely important culturally, commercialization also raises
concerns about the lack of recognition of and compensation for, the
intellectual property rights of indigenous peoples from Alaska to British
Columbia and Oregon.
The purpose of this paper is to clarify devil’s club’s
medicinal properties by summarizing reported traditional medicinal
applications, examining contemporary use by indigenous and non-indigenous
peoples, and reviewing recent phytochemical research. Intellectual property
rights and cultural and conservation issues associated with the
commercialization of this plant are also discussed.
Ethnobotanical Records of Traditional Use
A review of published and unpublished ethnographic sources reveals great
diversity in the uses of devil’s club among many indigenous groups over a wide
geographic area. These applications are summarized here in 34 broad categories
of medicinal use (Table 1), and eight categories of spiritual use (Table 2). The
indigenous peoples who use devil’s club include 38 linguistic groups from across
northwestern North America (Tables 1-2), representing nine language families.14
The region delineated by this cultural usage almost directly parallels the geographic
distribution of devil’s club (Figure 2) and underscores the cultural importance
of this plant across its range. In some areas devil’s club is used well outside
of its geographic range; the few areas where cultural use is not recorded from
within the area of its distribution appear to represent gaps in the ethnobotanical
documentation and not in actual usage.
Table 1: Summary of Medicinal Uses of Devil’s Club (Oplopanax horridus)
| Medicinal Uses |
Cultural Linguistic Group (and References) |
| Appetite Stimulant Infusion of inner bark. |
Nlaka’pamux (64); Secwepemc (65); Squamish (66) |
| Arthritis / Rheumatism Infusion or decoction of inner bark,
pounded leaves and sometimes roots, inner bark used in bath/steam bath, inner
bark chewed, crushed root used as poultice, and whole stems used to beat rheumatic
limbs as counter-irritant. |
Alutiiq (67, 68, 69, 70); Carrier (71); Ditidaht (72, 73); Gitxsan (17, 74,
75); Haida (2, 76, 77, 78, 79, 80, 81); Halkomelem (82); Hanaksiala (83); Makah
(84); Oweekeno (85); Nuu-chah-nulth (72); Stl’atl’imx (85); Nuxalk
(76, 77, 79); Sahaptin (86); Sechelt (72, 87, 88); Sekani (89); Squamish (66,
72); Stl’atl’imx (85, 90); Tlingit (2, 80); Tsimshian (80); Unspecified
(91) |
| Birth Control Decoction of roots. |
Métis (92) |
| Blood Purifier Decoction of inner bark. |
Carrier (73); Nlaka’pamux (64, 93) |
| Broken Bone Decoction of inner bark. |
Alutiiq (70); Gitxsan (95); Haida (2) |
| Cancer Infusion of inner bark. |
Alutiiq (70); Gitxsan (17); Haida (2; 81); Tlingit (2); Tsimshian (80) |
| Childbirth / Menstruation Inner bark mashed and swallowed,
or decoction of inner bark taken as purgative to expel afterbirth, to start post-partum
menstrual flow, regulate menstruation, and for cramps. |
Alutiiq (94); Carrier (77, 101); Hanaksiala (83); Lushootseed (86); Makah
(86); Secwepemc (96); Tlingit (16) |
| Diabetes Infusion or decoction of inner bark and sometimes
roots, both alone and in mixtures. |
Cree (92); Haida (81); Halkomelem (97); Heiltsuk (27); Metis (92); Nlaka’pamux
(64); Nuxalk (98); Sechelt (99); Secwepemc (65); Squamish (66); Stl’atl’imx
(85); Straits Salish (97, 100); Tsimshian (23) |
| Diphtheria Infusion of roots applied externally. |
Sekani (89) |
| Emetic / Purgative Decoction or infusion of inner bark prepared
in water or seal oil, both alone and in mixtures, roots chewed and the inner bark
sometimes swallowed. |
Alutiiq (68, 70); Carrier (3, 101); Eyak (3, 102); Gitxsan (79, 95); Haisla
(83); Haida (76); Makah (86); Nuxalk (77, 79, 98); Tlingit (3, 16, 103); Tsetsaut
(3); Unspecified (104); Wet’suwet’en (95) |
| Fertility Unspecified. |
Unspecified (104) |
| Fever Decoction of inner bark. |
Tanaina (105); Unspecified (104) |
| Flu Infusion of inner bark, alone and in mixtures, and the
inner stem bark chewed. |
Alutiiq (68, 70); Gitxsan (17); Haida (80, 81); Nlaka’pamux (4, 64);
Tanaina (106); Tsimshian (80); Tlingit (16, 80); Wet’suwet’en (107) |
| Gall Stones Infusion of inner bark. |
Haida (2); Tlingit (2) |
| Haemorrhaging and Blood Disorders Infusion of inner bark,
alone and in mixture, and berries pounded into paste taken internally. |
Comox (108); Hanaksiala (83) |
| Heart Disease Berries pounded into paste taken internally.
|
Alustiiq (70); Hanaksiala (83); Wet’suwet’en (107) |
| Insanity Introduced into the system by beating with stems.
|
Haida (80); Tsimshian (80); Tlingit (80) |
| Internal Infections Infusion of inner bark. |
Haida (80); Tanaina (106); Tsimshian (80); Tlingit (80); Unspecified (91) |
| Laxative Infusion or decoction of inner bark prepared both
alone and in mixtures. |
Gitxsan (109); Haida (2, 80); Haisla (83); Hanaksiala (83) Heiltsuk (4); Kwakwaka’wakw
(110); Nlaka’pamux (93; 64); Nuxalk (77); Tanaina (105); Tlingit (2, 80,
103); Tsimshian (80, 111); Unspecified (91) |
| Lice and Dandruff Pounded berries rubbed on hair and scalp.
|
Haida (78); Oweekeno (83) |
| Lymph Trouble (Dropsy) Ash of inner bark. |
Alutiiq (94) |
| Measles Decoction of inner bark. |
Halkomelem (72); Tlingit (106) |
| Pain Relief, Analgesic Decoction of inner bark, inner stem
bark mixed with oil and eaten, dried inner bark laid into tooth cavity, steam
bath with inner bark. |
Alutiiq (67, 69); Gitxsan (75); Haida (2); Kwakwaka’wakw (110, 112,
113, 114); Nuxalk (98); Oweekeno (83); Tlingit (2); Tsimshian (2) |
| Perfume, Baby Talc Unspecified. |
Makah (86) |
| Pneumonia Decoction or infusion of inner bark, and inner
bark used in steam baths with a variety of additional plants. |
Alutiiq (68); Squamish (66); Tlingit (16) |
| Respiratory Ailments, Coughs, Colds Decoctions and infusions
prepared from inner stem bark, whole stems and sometimes roots, inner bark also
chewed, used in sweat baths, and burned and dampened and worn around the neck.
|
Alutiiq (67, 70); Eyak (102); Gitxsan (17, 74); Haida (2, 80, 81); Halkomelem
(72); Hanaksiala (83); Okanagan (116); Oweekeno (83); Nlaka’pamux (4);Okanagan
(115); Sahaptin (86); Secwepemc (65); Squamish (66); Tagish (117); Tanaina (105);
Tlingit (2, 80); Tsimshian (80); Unspecified (5, 91, 104); Wet’suwet’en
(107) |
| Skin Wash Infusion or decoction of roots used as a general
wash for acne, skin disease, dandruff, etc. |
Alutiiq (70); Comox (108); Gitxsan (109); Sechelt (72); Sekani (89); Tlingit
(16) |
| Sores (Swellings, Cuts, Boils, Burns, and External Infections)
Inner bark, or infusion of, used externally as a poultice or wound dressing or
rubbed over sore, dried inner bark pulverized with pitch or burnt to ash and mixed
with oil or grease (sometimes salmonberries and dog feces) and applied externally,
berries pounded into a paste and applied externally, decoction of root applied
externally, and sliver of bark placed in wound to prevent infection. |
Alutiiq (67, 70, 94, 102); Carrier (71); Eyak (68); Gitxsan (17, 74, 109);
Haida (2, 3); Hanaksiala (83); Kwakwaka’wakw(113); Makah (84); Nlaka’pamux
(93); Nuxalk (98); Sechelt (99); Tanaina (105);Tlingit (2, 3, 6, 16, 103, 118);
Tsimshian (80); Unspecified (26, 91); Wet’suwet’en (74) |
| Stomach Trouble / Pains, Ulcers Infusion or decoction of
inner bark or paste made from berries taken internally. |
Gitxsan (17); Haida (2, 75, 76, 77); Hanaksiala (83); Kwakwaka’wakw
(110, 113, 114); Nlaka’pamux (64, 93); Nuxalk (76, 77, 79); Squamish (66);
Tanaina (105); Tlingit (2); Unspecified (26, 91) |
| Tonic Infusion or decoction of inner bark or sometimes roots,
inner bark chewed, and bark ash infused. |
Ditidaht (73); Gitxsan (17, 74); Haida (2, 77); Halkomelem (72); Nlaka’pamux
(64, 93); Nisga’a (75); Nuu-chah-nulth (119, 120); Oweekeno (83); Tlingit
(2, 16); Sechelt (99); Unspecified (91, 104); Wet’suwet’en (107) |
| Unspecified Use, General Sickness Unspecified. |
Alutiiq (67); Carrier (121); Ktunaxa (122); Gitxsan (75); Nlaka’pamux
(4); Nuxalk (88); Oweekeno (83); Quileute (123); Sechelt (99); Tlingit (124);
Tsimshian (3) |
| Venereal Disease Decoction prepared from inner bark and whole
stems both alone and in mixtures with a variety of other plants. |
Gitxsan (95); Haida (80); Tlingit (16, 80, 103); Tsimshian (80); Unspecified
(91) |
| Vision / Blindness Infusion of inner bark taken internally,
inner bark applied externally with pitch, and decoction used asan eyewash to reverse
the effects of cataracts. |
Haida (80); Hanaksiala (83); Tsimshian (80); Tlingit (80) |
| Weight Loss Infusion of de-spined stems. |
Nlaka’pamux (4, 64) |
Among all of the traditional medicinal uses of devil’s club
(Table 1), its most widespread is for the treatment of external and internal
infections, including tuberculosis. The efficacy of many of the treatments is
undoubtedly related to devil’s club’s significant antibacterial,7,11 antimycobacterial
(active against bacteria in the genus Mycobacterium),10,11 antifungal,8,11 and
antiviral properties.9,15 Devil’s club is also commonly used by many
cultural groups to treat arthritis, rheumatism, respiratory ailments, and as an
emetic and purgative. It is also used as an aid in childbirth (post-partum),
for internal hemorrhaging, as an analgesic, to treat stomach and digestive
tract ailments, broken bones, fever, dandruff, lice, headaches, and as a
treatment for cancer. Several parts of the shrub, including inner bark, inner
bark ash, whole stems, roots, berries, and leaves, are used in a variety of
ways to effect these treatments. However, the most common type of preparation
is as an infusion or decoction of the stem inner bark.
In addition to ethnographic accounts of medicinal uses, there are also numerous
sources that describe spiritual applications of devil’s club. These include purification
and cleansing; protection against supernatural entities, epidemics and evil influences;
acquisition of luck; to combat witchcraft; as ceremonial and protective face paint;
and in rituals by shamans and others to attain supernatural powers (Table 2).
Two of the most widespread spiritual uses are bathing with a devil’s club inner
bark solution for personal protection and purification, and its use, particularly
the spiny or de-spined aerial stems, as an amulet for protection against a variety
of external influences (Table 2). External and internal cleansing involving the
use of devil’s club “was, and is, of paramount importance” to many of the cultural
groups throughout devil’s club’s range.4 The inner stem bark of devil’s
club has also often been used in solution to wash down fishing boats, fishnets,
and to purify a house after an illness or death, and, as charcoal, to prepare
protective face paint for ceremonial dancers (Table 2). John Thomas explained
that amongst the Ditidaht, and many other neighboring groups, devil’s club is
considered sacred and “along with red ochre paint is considered to be a link between
the ordinary, or profane world, and the supernatural, or spirit world.”4
Although it is useful for the purposes of this paper, an explicit division between
medicinal and spiritual uses of devil’s club does not reflect traditional conceptions
of health and healing4,16,17 and most “medicinal” applications of devil’s
club are inextricably linked to “spiritual” applications of the plant, particularly
its use for cleansing and purification. Devil’s club also figures significantly
in the traditional narratives of many cultural groups throughout its range.14
Table 2: Summary of Spiritual Uses of Devil’s Club (Oplopanax horridus)
| Spiritual Uses |
Cultural Linguistic Group (and References) |
| End Bad Weather Unspecified |
Eyak (102); Tlingit (16) |
| Luck Wood retained for luck, bark used in bath, and rubbed
on body, and fresh bark chewed. |
Eyak (102); Gitxsan (17, 109); Haida (125, 126, 127); Haisla (83); Hanaksiala
(83); Tlingit (16); Tsimshian (83, 128); Wet’suwet’en (74) |
| Paint Charcoal, sometimes mixed with bear grease used for
used as medium for paint mixed with berries. |
Ditidaht (73); Haisla (83); Hanaksiala (83); Nuu-chah-nulth (119,face paint
used in ceremonies and for protection. Bark also 120); Secwepemc (65); Squamish
(66); Straits Salish (100) |
| Personal Purification Infusion of inner bark and inner bark
used in bath. |
Eyak (102); Gitxsan (17, 74, 109); Haida (125, 126); Haisla (83);Hanaksiala
(83); Tlingit (3, 118); Tsimshian (4, 83); Wet’suwet’en (74, 107); |
| Protection Bark and stems used as an amulet, charcoal Aused
for protective face paint, bark used in bath for protection, hoop of stem steeped
through for protection against supernatural entities, epidemics, evil influences,
love charms and shamans’ spells, and inner bark sewn into pouch and worn
around the neck as an amulet. |
lutiiq (70); Ditidaht (73); Eyak (102); Gitxsan (21); Haida (78); Haisla (83);
Hanaksiala (83); Nisga’a (75); Nuu-chah-nulth (119,120); Nuxalk (79); Sekani
(89); Tagish (117); Tlingit (17); Tsimshian (83) |
| Purification of House Inner bark burned as a fumigant, placed
in pouches, under pillows, or used with other plants to prepare an infusion to
purify a house, often following a death. |
Eyak (102); Gitxsan (17, 75); Haisla (83); Hanaksiala (83); Nuxalk (88); Sekani
(89); Wet’suwet’en (74, 107) |
| Shamanic Infusion of inner bark or roots, and roots chewed.
|
Blackfoot (129); Eyak (102); Haisla (83); Hanaksiala (83); Oweekeno (83);
Sekani (89); Tlingit (16, 124); Tsimshian (83) |
| Witchcraft Prophylactic against witchcraft. |
Alutiiq (130); Hanaksiala (83); Tlingit (16, 118) |
Contemporary Use in the Herb and Dietary Supplement Industry
Many of devil’s club’s uses in herbal medicine parallel its
most commonly documented traditional uses (Table 1). Overall though, the modern
commercial applications of devil’s club in the North American herbal market are
for treating a smaller number of health problems and lack the spiritual
practices associated with traditional use. Western herbalists report that the
roots of devil’s club (and to a lesser extent the inner stem bark) are a strong
respiratory stimulant and expectorant18,19 and recommend their use
for rheumatoid arthritis and other autoimmune conditions,18,20 as
well as to treat eczema, sores, and a number of internal and external
infections.21 Devil’s club is also commonly recommended for the
treatment of type II adult onset diabetes,18,22 a use of devil’s
club that is also extensive in indigenous communities. However, since there is
considerable risk and uncertainty associated with such therapy, such
recommendations should be viewed with caution.21 In one notable
case, devil’s club is recommended as a pancreatic tonic that is purported to
help lower blood sugar levels by increasing the efficiency of insulin
production in the pancreas.22 In this and other similar cases it is
unclear if the reasoning given is based on the clinical evidence, which is
equivocal and conflicting.21 Early clinical research on devil’s
club, inspired by its widespread use by indigenous peoples for adult diabetes,
reported that a white precipitate isolated from extracts of devil’s club “root
bark” exhibited a hypoglycemic effect in lab hares.23 Subsequently,
in experiments involving two human subjects, Justice2 presented some
additional evidence to support the hypoglycemic activity of devil’s club’s root
and stem bark. However, additional work by Piccoli et al.,24 Sturr
et al.,25 and Thommasen et al.26 using extracts from
“root bark,” and research by MacDermot27 using a decoction of stems,
provide data that do not substantiate the hypoglycemic activity reported
previously. Since devil’s club is still widely, and increasingly, used as a
treatment for late onset type II diabetes and is listed in a recent review of
antidiabetic plants,28 additional research and more rigorous
clinical trials are required to validate and characterize or to disprove
hypoglycemic properties in devil’s club.
Notable commercial applications of devil’s club that depart
from traditional use include marketing strategies portraying devil’s club as
“Pacific ginseng,” “Alaskan ginseng,” or “wild armored Alaskan ginseng.”12,13
In these cases, and on occasion when devil’s club is promoted under its
standardized common name, products are marketed on the basis of purported
adaptogenic and tonic properties. Suggestions of its use as an alternative to
ginseng parallel those of eleuthero (Eleutherococcus senticosus Maxim., Araliaceae), formerly called “Siberian
ginseng.” Devil’s club is increasingly used as a ginseng substitute in marketed
herbal formulas and is considered to have significant potential in U.S. and
Asian markets in this role.12 Although devil’s club is used as a
tonic in some traditional medicinal applications (Table 1), its properties as
an adaptogen are by no means its most distinct. The belief of some herbalists
that devil’s club can be readily substituted for ginseng, with similar effects
and benefits, has not been demonstrated empirically. Indeed, devil’s club’s
emetic and purgative properties are often what some first-time users highlight
when describing their experience with the plant (possibly due to ingestion of
relatively high doses). Furthermore, any pharmacological and therapeutic
similarities devil’s club shares with ginseng are not well documented, and a
number of authors stress that these plants should not be used as if they were
the same.19,21 Additional applications that are unconnected to
traditional indigenous use and appear to have no empirical basis include
treatment for hyperthyroidism, for impotence, and as a phytoestrogen.21
Another notable divergence between traditional and
contemporary commercial applications of devil’s club relates to the plant parts
used. Most of the devil’s club products currently available commercially,
including devil’s club teas, tinctures, capsules, and formulas that contain
devil’s club, contain “root bark” as the main ingredient. “Root bark” is also
one of the most common plant parts sold as a crude drug. Unlike the traditional
use of devil’s club, which primarily involves inner bark of the aerial stems
(Table 1),14 the commercial use of devil’s club “root bark” seems to
be driven by perceived superior potency of roots. Like the marketing of devil’s
club under alternate common names that equate it with ginseng, the sale of
devil’s club “root bark” appears also to be an attempt to highlight devil’s
club’s relationship to ginseng, since the root of the latter is used
medicinally. Any therapeutic advantage of the roots over the stem bark, like
the marketing of devil’s club as a ginseng substitute, appears largely
unsubstantiated by research, much of which has been conducted using stem inner
bark. Furthermore, the commercial harvesting of devil’s club roots in
preference to stems has some important conservation implications. Devil’s club
is a shallow rooting, long-lived, clonal shrub that expands vegetatively
through the layering of horizontal, or decumbent stems, and rarely by seedling.1
Like many medicinal plants, devil’s club is not currently cultivated in any
significant quantity and is almost exclusively wild-harvested. Although devil’s
club can be wild-crafted sustainably,14,29 the large-scale harvest
of its interconnected clonal fragments may have an adverse impact on the
persistence of populations.
Phytochemistry and Biological Activity
Phytochemical information on the active constituents of
devil’s club is somewhat confounded by varying taxonomic treatments of the
genus Oplopanax. Most authorities treat Oplopanax
as a genus made up of three species: O.
elatus Nakai in Russia and Korea;30 O.
japonicus (Nakai) Nakai in Japan;31
and O. horridus in North America.32
Throughout this paper we have adopted this treatment and refer to O.
horridus in this strict sense when
employing the common name devil’s club. However, there are other authorities
that treat all three as subspecies of O. horridus.33,34 Since most research has been
conducted on either the Russian or Japanese species (or subspecies) of Oplopanax, with little published descriptive phytochemical
work on devil’s club, it is unclear if many of the active constituents commonly
cited as components of devil’s club are actually present in North American
devil’s club, or only in the Russian and Japanese species. For example, Moore18
lists nerolidol, torreyol, dodinene, busnesol, dodecenol, cadene, and
cerdrol as constituents of devil’s club. However, since this research was
conducted on Oplopanax elatus [O.
horridus ssp. elatus]35 it is unclear if it is applicable to
the North American populations of O. horridus [O. horridus ssp. horridus].
 Another
group of compounds also commonly cited as constituents of devil’s club (O.
horridus) are saponins (triterpenoid glycosides).36 Although a
number of triterpenoids have been described from O. elatus and O. japonicus,37-44
to date none have been described from North American devil’s club. Although it
is likely that North American devil’s club does contain some of the same compounds
as these close relatives, presently the only published report to support this
describes glycosidal principles of a possible saponin-like nature.25
There is also no evidence that devil’s club contains a specific group of saponins
known as the ginsenosides as is often claimed and used to assert its
adaptogenic properties and therapeutic similarity to ginseng.18 Additionally,
none of the saponins described in devil’s club relatives O. elatus and
O. japonicus are ginsenosides (saponins with dammarane type triterpenoid aglycones45).
According to Zhuravlev and Kokyada,46 O. elatus and a larger
group of Russian Araliaceae species (including E. senticosus<
do not contain ginsenosides, but rather saponins that have aromatic
triterpenoid aglycones. Another
group of compounds also commonly cited as constituents of devil’s club (O.
horridus) are saponins (triterpenoid glycosides).36 Although a
number of triterpenoids have been described from O. elatus and O. japonicus,37-44
to date none have been described from North American devil’s club. Although it
is likely that North American devil’s club does contain some of the same compounds
as these close relatives, presently the only published report to support this
describes glycosidal principles of a possible saponin-like nature.25
There is also no evidence that devil’s club contains a specific group of saponins
known as the ginsenosides as is often claimed and used to assert its
adaptogenic properties and therapeutic similarity to ginseng.18 Additionally,
none of the saponins described in devil’s club relatives O. elatus and
O. japonicus are ginsenosides (saponins with dammarane type triterpenoid aglycones45).
According to Zhuravlev and Kokyada,46 O. elatus and a larger
group of Russian Araliaceae species (including E. senticosus<
do not contain ginsenosides, but rather saponins that have aromatic
triterpenoid aglycones.
The earliest, and some of the only, published descriptive
phytochemical work that has been conducted on the North American devil’s club
is by Kariyone and Morotomi,47 who described a sesquiterpene
(equinopanacene) and a sesquiterpene alcohol (equinopanacol) in O. horridus. In more recent phytochemical investigations on O.
horridus, Bloxton et al.48 reported
a number of sterols and four sesquiterpenes, one of which (spatulenol) is novel
to the genus. Kobaisy et al.11 described two novel and three
previously described polyenes, one of which (oplopandiol) has recently been
synthesized.18 These acetylenes all display significant
antimycobacterial and antifungal activity49 and most are active
against common bacteria such as Staphylococcus aureus, Bacillus subtilis, Pseudomonas aeruginosa, Escherichia coli, and Candida albicans. These compounds are also active against Mycobacterium
tuberculosis and Mycobacterium
avium, both of which can cause significant
clinical tuberculosis, particularly virulent in immuno-compromised hosts, AIDS
patients being especially vulnerable. Notably, these pathogens are also
responsible for the epidemic status of tuberculosis in Canada’s indigenous
population.50 Since many strains of M. tuberculosis and M. avium are also resistant to the most commonly used antimycobacterial drugs,
there is considerable interest in the potential of devil’s club in tuberculosis
therapy. Extracts of devil’s club inner bark also partially inhibit a
respiratory syncytial virus.9
In other research, immunologist George Luciuk has claimed
that devil’s club has anti-microbial effects on upper and lower respiratory
tract infections15. In related investigations on the Russian
species, Me et al.35 reported that essential oils derived from O.
elatus show antifungal activity against
five pathogenic species: Microsporum gypseum, M. lanosun, Trichophyton gypseum, T. purpureatum, and Epidermophyton floccosum. Aihua et al.51 also report that O. elatus shows anti-mycotic action against a number of common
pathogenic fungi. Additionally, a sesquiterpene, a sesquiterpene alcohol, and a
sesquiterpene ketone have been isolated from >O. japonicus; a
derivative of the latter is used in Japan in commercial preparations to treat
coughs and colds.52
Cultural Concerns and Intellectual Property Rights
In 1992, signatories to the United Nations Convention on
Biological Diversity (Biodiversity Convention) formally recognized the
intellectual property rights of the Earth’s indigenous and traditional peoples
in Articles 1 and 8j by calling for fair and equitable sharing of any benefits
resulting from the sustainable use of biodiversity and traditional and local
knowledge.53,54 As of September 2003, 187 countries were party to
the convention. In the case of devil’s club and many other culturally important
plants, it is clear that commercial medicinal applications and research
interest in pharmaceutical applications are based directly on traditional
knowledge and use drawn mainly from the ethnobotanical record. Consequently
there is a clear conflict between current commercialization efforts and
articles 1 and 8j of the Convention.55
 Since
Canada is a signatory to the Biodiversity Convention, it is legally and morally
bound to uphold all 40 articles. However, despite the obvious conflict between
the commercialization of devil’s club in the absence of compensation, and Articles
1 and 8j of the Biodiversity Convention, there are no effective legal means to
ensure that the compensation called for in the Convention is provided or negotiated.
The concept of copyright and patent protection fundamental to Western intellectual
property rights law is designed to protect corporations and individual entrepreneurs
and, thus far, has been inadequate to address intellectual property rights that
relate to knowledge held in common by one or several cultural groups.56
In the case of many traditionally used medicinal plants for which detailed ethnobotanical
information has been recorded and published, protection of intellectual property
rights is particularly problematic. Under current law, traditional knowledge that
has been published in the ethnographic literature is considered within the public
domain, and consequently as information that is ineligible for conventional intellectual
property rights protection. Concern about the lack of protection for knowledge
that is now a part of the public domain is described in more detail in the Report
of the Royal Commission for Aboriginal Peoples.57 Since
Canada is a signatory to the Biodiversity Convention, it is legally and morally
bound to uphold all 40 articles. However, despite the obvious conflict between
the commercialization of devil’s club in the absence of compensation, and Articles
1 and 8j of the Biodiversity Convention, there are no effective legal means to
ensure that the compensation called for in the Convention is provided or negotiated.
The concept of copyright and patent protection fundamental to Western intellectual
property rights law is designed to protect corporations and individual entrepreneurs
and, thus far, has been inadequate to address intellectual property rights that
relate to knowledge held in common by one or several cultural groups.56
In the case of many traditionally used medicinal plants for which detailed ethnobotanical
information has been recorded and published, protection of intellectual property
rights is particularly problematic. Under current law, traditional knowledge that
has been published in the ethnographic literature is considered within the public
domain, and consequently as information that is ineligible for conventional intellectual
property rights protection. Concern about the lack of protection for knowledge
that is now a part of the public domain is described in more detail in the Report
of the Royal Commission for Aboriginal Peoples.57
Since the United States and four other nations58
have not ratified the Convention on Biological Diversity, it is also unlikely
that the principles expressed in articles 1 and 8j will be used successfully to
protect intellectual property rights of indigenous peoples in the U.S.
Additional difficulties, discussed in more detail by Posey and Dutfield,55
Mann,59 and Bannister,60 include conflicts between
indigenous world-views regarding ownership of knowledge and the monopolistic
and exclusionary axioms inherent in the Western concept of intellectual
property rights.55, 57
The summary of traditional uses of devil’s club recorded in the ethnobotanical
record that is presented here (Figure 2; Tables 1-2) makes the origins of this
knowledge unequivocal. Thus, despite the failure of current legal mechanisms to
compensate the original users of this plant, current practice is clearly at odds
with the ethical framework embodied in the Biodiversity Convention and numerous
other international agreements. Are governments where devil’s club is harvested
and sold commercially ethically and/or legally obligated to ensure that consultation
and compensation regarding commercialization is undertaken? Moreover, what are
the ethical obligations of harvesters, wholesalers, users, practitioners, journalists,
and researchers involved in the commercial use of devil’s club and other medicines
whose commercial applications are rooted in traditional knowledge?
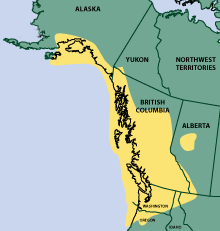
Figure 1. Geographic distribution of devil’s club Oplopanax horridus
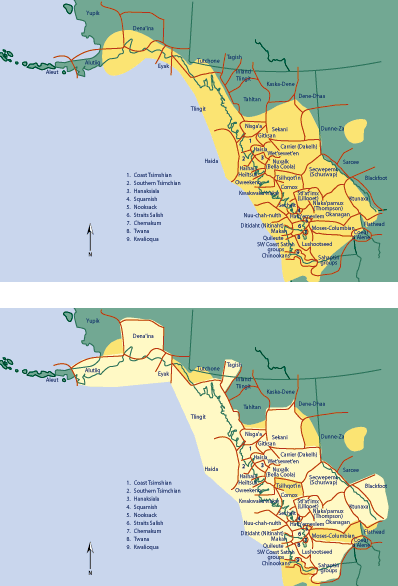
Figure 2. Geographic distribution of devil’s club (dark yellow on the
upper map) in relation to the area of cultural use and linguistic recognition
(pale yellow on the lower map) in Western North America. (Figure modified from
62.)
The bulk of devil’s club use in the herbal industry
parallels its commonly described traditional uses. However, in many instances it is unlikely that
the commercialization of these uses would be considered culturally appropriate.61
Without consultation it is doubtful that commercial exploitation of a plant of
such enormous medicinal and spiritual importance to indigenous peoples would be
supported by its original users. Like many culturally important medicinal
plants, devil’s club is thought by many Indigenous Peoples to lose its healing
efficacy if its use is publicized too widely. One of the mostly striking
examples of potentially inappropriate commercialization of devil’s club is the
web-based sale of ready-made devil’s club ‘spirit paint.’62 Given
the spiritual importance and sacredness of devil’s club and red ochre paint,
its sale would likely be seen by many indigenous people as an inappropriate
imitation of indigenous knowledge.14,60
Conclusions
Devil’s club, a highly significant species for indigenous
peoples of western North America, is currently being harvested and marketed as
a ginseng-like herbal medicine. Although it is related to ginseng, there is
little conclusive research to demonstrate that it has ginseng’s adaptogenic and
immune-enhancing properties. There is however, extensive phytochemical evidence
that supports devil’s club’s widespread use to treat internal and external
infections, in particular its potential to control Mycobacterium spp. It is also commonly reported that devil’s club
has hypoglycemic properties and as such, it is used by indigenous and
non-indigenous peoples alike to treat type II late onset diabetes. However, its
efficacy as a treatment for this condition has not been clinically
demonstrated. Furthermore, commercial development of devil’s club has not been
in keeping with the United Nations Convention on Biodiversity and a number of
additional international agreements. To date, little or no consultation has
been undertaken with the indigenous peoples from whom knowledge of devil’s
club’s properties has been derived, and no provisions have been made for
compensation or benefit sharing with the original users of devil’s club.
Additionally, indigenous peoples and others have raised concerns that
commercial harvesting of devil’s club, especially the roots, will compromise
its ability to persist in some localities within its range. Before the
commercial harvest and marketing of devil’s club is expanded any further, it is
imperative that researchers and promoters work in consultation with the
indigenous users of this culturally significant shrub to determine (1)
appropriate (e.g., ethical) harvesting methods and applications, (2) active
pharmacological compounds, and (3) modes of production that are sustainable and
that conform to the requirements of the Biodiversity Convention and other
international protocols. Until these steps have been accomplished, the
commercialization of devil’s club is at best problematic.
Acknowledgements
The authors wish to acknowledge the contribution of the
following individuals to the development of this paper: Geraldine Allen, Sean
Barnes, Arvid Charlie, Don Eastman, Amanda Howe, Allison McCutcheon, Stephanie
Mills, Karen Robin, Bill White, and Nan Vance. Three anonymous reviewers also
provided helpful feedback on the manuscript. Financial support for this project
has been provided by the National Science and Engineering Research Council, PGS
A Scholarship (220697) to Trevor Lantz; Global Forest Research, Field Research
Grant (GF-18-2000) to Trevor Lantz and Nancy Turner; to Haida Artist Giitsxaa
(Ron Wilson) who contributed the painting of Devil’s Club Man; and the Canadian
Forest Service, First Nations Forestry Program Grant (00-1-188) to the
Hupacasath First Nation.
Authors’ Biography
Trevor Lantz is pursuing his Ph.D. in the Centre for
Applied Conservation Research at the University of British Columbia. He
completed his B.Sc. in Botany in 1998 at the University of Alberta, and his
masters, which focused on the ecology and ethnobotany of devil’s club, in 2001
at the University of Victoria.
Kristina Swerhun was
born in Scarborough, Ontario and moved to British Columbia in 1996 where she
completed her B.Sc. in Biology in 2000. She has worked as a field biologist
throughout the Pacific Northwest and now lives in Whistler, BC.
Nancy Turner is an ethnobotanist and professor in the
School of Environmental Studies, University of Victoria. For over 30 years she
has worked with indigenous elders and plant specialists in British Columbia,
documenting traditional botanical knowledge.
References
1. Lantz TC, Antos JA. Clonal expansion in the deciduous
understory shrub, devil’s club (Oplopanax horridus (Sm.) Torr. and A. Gray ex. Miq.). Canadian
Journal of Botany 2002;80:1052-62.
2. Justice JW. Use of devil’s club in southeast Alaska. Alaska
Med 1966;8(2):36-9.
3. Smith
GW. Arctic pharmacognosia. Arctic 1973;26:324-33.
4. Turner
NJ. Traditional use of devil’s club (Oplopanax horridus: Araliaceae) by native peoples in western North
America. Journal of Ethnobiology
1982;2:1-11.
5. Gottesfeld
LMJ. The importance of bark products in the aboriginal economies of Northwestern
British Columbia, Canada. Economic Botany 1992;46:148-57.
6. Blaschke
E. Topographia Medica Portus Novi-Archangelscensis. Wiehoberi, St. Petersburg; 1842.
7. McCutcheon
AR, Ellis SM, Hancock REW, Towers, GHN. Antibiotic screening of medicinal plants
of the British Columbian native peoples. J Ethnopharmacol 1993;37:213-23.
8. McCutcheon
AR, Ellis SM, Hancock REW, Towers, GHN. Antifungal screening of medicinal
plants of the British Columbian native peoples. J Ethnopharmacol 1994;44:157-69.
9. McCutcheon
AR, Roberts TE, Gibbons E, et al. Antiviral screening of British Columbian
medicinal plants. J Ethnopharmacol
1995;49:101-10.
10. McCutcheon
AR, Stokes RW, Thorson LM, et al. Anti-mycobacterial screening of British
Columbian medicinal plants. International Journal of Pharmacognosy 1997;35:77-83.
11. Kobaisy
M, Abramowski Z, Lermer L, Saxena G, Hancock REW, Towers GHN. Antimycobacterial
polyenes of devil’s club (Oplopanax horridus), a North American native medicinal plant. J Nat Prod 1997;60:1210-13.
12. Wills
RM, Lipsey RG. An economic strategy to develop non-timber forest products
and services in British Columbia. Final
Report. Victoria, B.C.: Forest Renewal B.C.; 1999. Project No.: PA97538-ORE.
13. Awang
DVC. What in the name of Panax are those
other ginsengs? HerbalGram 2003;57:35.
14. Lantz
TC. Population ecology and ethnobotany of devil’s club (Oplopanax horridus (Sm.) Torr. and A. Gray. ex. Miq. [master’s thesis].
Victoria, B.C.: University of Victoria; 2001.
15. Wigod
R. A Plant that Heals. The Vancouver Sun
1998 March 2; Sect. B:10.
16. de
Laguna F. Under Mount St. Elias: history and culture of the Yakutat Tlingit. Smithsonian Contributions to Anthropology 7.
Washington (DC): Smithsonian Institution Press; 1972
17. Johnson
LM. Health, wholeness and the land: Gitksan traditional plant use and
healing [dissertation]. Edmonton (AB):
University of Alberta; 1997.
18. Moore
M. Medicinal plants of the Pacific West.
Santa Fe (NM): Red Crane Books; 1993.
19. Tilford
GL. Edible and medicinal plants of the West.
Missoula (MT): Mountain Press Publishing Company; 1997.
20. Willard
T. Edible and medicinal plants of the Rocky Mountains and neighboring
territories. Calgary (AB): Wild Rose
College of Natural Healing; 1992.
21. Howe
A. What does the herbalist need to know about devil’s club (Oplopanax horridus) before incorporating
this plant into the materia medica? A review of the traditional and scientific
literature, commercial claims and ethical considerations [master’s thesis]. University of Wales, U.K.; 2003.
22. Green
J. The male herbal: health care for men & boys. 7th ed. Berkeley
(CA): Crossing Press; 1991.
23. Large
RG, Brocklesby HN. A hypogylcemic substance from the roots of devil’s club (Fatsia
horrida). CMAJ 1938;39:32-35.
24. Piccoli
LJ, Spinapolice ME, Hecht M. A pharmacologic study of devil’s club root (Fatsia
horrida). J Am Pharm Assoc 1940;29:11-12.
25. Stuhr
ET, Henry FB. An investigation of the root bark of Fatsia horrida. Pharmaceutical Archives 1944;15:9-15.
26. Thommasen
HV, Wilson RA, McIlwain RG. Effects of devil’s club tea on blood glucose in
diabetes mellitus. Can Fam Physician
1990;36:62-5.
27. MacDermot
JH. Food and medicinal plants used by the Indians of British Columbia. CMAJ 1949;61:177-83.
28. Marles
RJ, Farnsworth NR. Antidiabetic plants and active constituents. Phytomedicine 1995;2:137-89.
29. Drum
R. Devil’s club, Oregon grape, chaparral: three traditional western herbs in
contemporary herbal practice. Partner Earth Education Center Website. Available
at: . Accessed
October 2, 2003.
30. Skiskin
BK, ed. Flora of the U.S.S.R. Moscow (Russian Federation): Botanical Institute of
the Academy of Sciences of the U.S.S.R [Translated by Israel Program for
Scientific Translation, Jerusalem (Israel)]; 1950.
31. Ohwi
J. Flora of Japan. Washington (D.C.):
Smithsonian Institution Press; 1965.
32. Hitchcock
LC, Cronquist A, Owenbey M, Thompson JW. Vascular plants of the Pacific
Northwest. Seattle (WA): University of
Washington Press; 1973.
33. Hultén
E. The flora of Alaska and neighboring Territories. Stanford (CA): Stanford University Press; 1968.
34. Scoggan
HJ. The flora of Canada. Ottawa (ON):
National Museums of Canada; National Museum of Natural Sciences Publications in
Botany No.7; 1979.
35. Me
HM, Li CG, Su ZW, Wang NP, Zhao JX, Jiang YG. 1987. Studies on chemical
constituents and antifungal activities of essential oil from Oplopanax
elatus Nakai [in Chinese]. Yao
xue xue bao [Acta Pharaceutica
Sinica] 1987;22:549-52.
36. Kershaw
LJ. Edible and medicinal plants of the Rockies. Edmonton (AB): Lone Pine Publishing; 2000.
37. Wang
GS, Zhao CF, Xu JD, Murayama T, Miyakoshi M, Junzo S. Studies on the glycosides
in the leaves of Oplopanax elatus Nakai.
Chemical Research in Chinese Universities
1994;10:185-92.
38. Wang
GS, Xu JD, Murayama T, Junzo S. Isolation and structure elucidation of new
glycosides from the leaves of Oplopanax elatus Nakai. III. Chinese Science Bulletin 1994;39:1969-72.
39. Wang
GS, Zhao CF, Xu JD, Murayama T, Junzo S. Isolation and structure elucidation of
new glycosides from the leaves of Oplopanax elatus Nakai (II). Chemical Research in Chinese
Universities 1994;10:285-90.
40. Wang
GS, Xu JD, Ma XL, Sun YX, Liu JZ, Murayama T, Shoji J. Chemical studies on
glycosides in the leaves of Oplopanax elatus Nakai. (IV). Chemical Research in Chinese Universities 1997;13:34-38.
41. Wang
GS, Xu JD, Murayama T, Shoji J. Isolation and structure elucidation of
cirensenosides Q and R [in Chinese]. Zhongguo Zhongya Zazhi [Chinese Journal of Materia Medica] 1997;22:101-03.
42. Hirai
Y, Murayama T, Miyakoshi M, et al. Three new lupane glycosyl esters from Oplopanax
japonicus leaves. Natural
Medicines 1995;49:462-67.
43. Wang
GS. Isolation and structure elucidation of cirensenosides O and P from the
leaves of Oplopanax elatus Nakai [in
Chinese]. Yao xue xue bao [Acta
Pharmaceutica Sinica] 1996;31:940-44.
44. Hongui
Z, Guangxuan W, Yongmao Z. Studies on chemical constituents from stems of Oplopanax
elatus Nakai [in Chinese]. Zhongguo
Zhongya Zazhi [Chinese Journal of
Materia Medica] 1993;18:104-5.
45. Bruneton
J. Pharmacognosy, phytochemistry, medicinal plants. 2nd ed. Paris, (France): Lavoisier Publishing;
1999.
46. Zhuravlev
N, Kolyada AS. Araliaceae: ginseng and other aralia of the Russian Far East.
Vladivosktok (Russia) [in Russian]: Dalnauka; 1996. English summary available
at:
.
Accessed September 25, 2003.
47. Kariyone
T, Morotomi S. The essential oil of Echinopanax horridus, Decne et Planch [in Japanese]. Yakugaku
zasshi [Journal of the
Pharmaceutical Society of Japan]
1927;546:671-74.
48. Bloxton
JD, Der Marderosian A, Gibbs R. Bioactive constituents of Alaskan devil’s root
(Oplopanax horridus, Araliaceae). Economic
Botany 2002;56:285-289.
49. Xu
L, Wu XH, Zheng GR, Cai JC. First total synthesis of optically active
oplopandiol acetate, a potent antimycobacterial polyyne isolated from Oplopanax
horridus. Chinese Chemical
Letters 2000;11:213-216.
50. Young
TK, Reading J, Elias B, O’Neil JD. Type 2 diabetes mellitus in Canada’s First
Nations: status of an epidemic in progress. CMAJ 2000;163:561-66.
51. Aihua
F, Honggui Z, Ling Z. Clinical and laboratory studies of the essential from Oplopanax
elatus Nakai against fungi [in Chinese]. Zhonghua
Pifuke Zazhi [Chinese Journal of
Dermatology] 1997;30:310-311.
52. Small
E, Catling PM. Canadian medicinal crops.
Ottawa (ON): NRC Research Press; 1999.
53. Reid
WV, Laird SA, Meyer CA, et al. Biodiversity prospecting: using genetic
resources for sustainable development. Washington
(DC): World Resources Institute; 1993.
54. Baker
JT, Borris RP, Carté B, Cordell GA, Soejarto DD, Cragg GM, Gupta MP, Iwu MM,
Madulid DR, Tyler VE. Natural product drug discovery and development: new
perspectives on international collaboration. J Nat Prod 1995;58:1325-57.
55. Posey
DA, Dutfield G. Beyond intellectual property rights: towards traditional
resource rights for indigenous peoples and local communities. Ottawa (ON): International Development Research
Center; 1996.
56. Greaves
T, ed. Intellectual property rights for Indigenous Peoples: a sourcebook. Oklahoma City (OK): Society for Applied
Anthropology; 1995.
57. Royal
Commission on Aboriginal Peoples (Canada). Ottawa, (ON): Report of the Royal
Commission on Aboriginal Peoples; 1996. Quoted by: Bannister KP. Chemistry
rooted in cultural knowledge: unearthing the links between antimicrobial
properties and traditional knowledge in food and medicinal plant resources of
the Secwepemc (Shuswap) Aboriginal Nation
[dissertation]. Vancouver (B.C.): University of British Columbia; 2000.
58. Convention
of Biological Diversity. Parties to the Convention on Biological Diversity /
Cartagena Protocol on Biosafety. Available online at:
. Accessed October 2, 2003.
59. Mann
H. Indigenous peoples and the use of intellectual property rights in Canada:
case studies relating to intellectual property rights and the protection of
biodiversity. Ottawa (ON): Intellectual
Property Policy Directorate Corporate Governance Branch Industry Canada; 1999.
60. Bannister
KP. Chemistry rooted in cultural knowledge: unearthing the links between
antimicrobial properties and traditional knowledge in food and medicinal plant
resources of the Secwepemc (Shuswap) Aboriginal Nation [dissertation]. Vancouver (B.C.): University of
British Columbia; 2000.
61. Turner
NJ. “Doing it Right”: Issues and practices of sustainable harvesting B.C.
Journal of Ecosystems and Management [serial
online]. 2001;1(1):1-11 Available online at: .
Accessed October 4, 2003. [Reprinted in United Plant Savers, Journal
of Medicinal Plant Conservation 2003;Winter:9-11].
62. Traditional
Healing Herbal Remedies of the Pacific Northwest First Nations
Tribes Site. Devil’s Club Root. Available online at:
. Accessed September
28, 2003.
63. Turner
NJ, Loewen DC. The original “free trade”: exchange of botanical products and
associated plant knowledge in northwestern North America. Anthropologica 1998;60:49-70.
64. Turner
NJ, Thompson LC, Thompson MT, York AZ. Thompson ethnobotany: Royal British Columbia Museum Memoir No.
3. Victoria (B.C.): Royal British Columbia
Museum; 1990.
65. Turner
NJ, Ignace MB, Loewen D. Plants of the Secwepemc People. Kamloops (B.C.) Secwepemc Cultural Education Society;
1998.
66. Bouchard
R, Turner NJ. Squamish ethnobotany.
Victoria, B.C.: British Columbia Indian Language Project; 1976.
67. Wennekens
AJ. Aleut botany in the Prince William Sound. Alaska Native News 1983;1(8):21-3.
68. Wennekens
AJ. Traditional plant usage by Chugach natives around Prince William Sound
and on the Lower Kenai Peninsula, Alaska
[master’s thesis]. Anchorage (AK): University of Alaska; 1985.
69. Stanek
RT. Patterns of wild resource use in English Bay and Port Graham, Alaska. Anchorage, (AK): Subsistence Division, Alaska
Department of Fish and Game; 1985. Technical Paper 104.
70. Russell
PN. English Bay and Port Graham Alutiiq plantlore. Anchorage (AK): Homer Society of Natural History, Chugach Heritage
Foundation, Alaska Native Plant Society; 1991.
71. Hebda
RJ, Turner NJ, Birchwater S, Kay M, the Elders of Ulkatcho. Ulkatcho food
and medicine plants. Anahim Lake, (B.C.):
Ulkatcho Publishing; 1996.
72. Rollins
D. Materia medica of the Northwest Coast Indians of British Columbia. Victoria (B.C.): British Columbia Indian Language
Project; 1972.
73. Turner
NJ, Thomas J, Carlson BF, Ogilvie RT. Ethnobotany of the Nitinaht Indians of
Vancouver Island. Victoria (B.C.): British
Columbia Provincial Museum, Victoria and Parks Canada, Western Region; 1983.
British Columbia Provincial Museum Occasional Papers Series No. 24.
74. Gottesfeld
LMJ, Anderson B. Gitksan traditional medicine: herbs and healing. Journal of
Ethnobiology 1998; 8:13-33.
75. Oates
MLB. Research on plants native to British Columbia. Final Report. Terrace, (B.C.); 1994.
76. Harrison
C. Ancient warriors of the North Pacific, the Haidas. London (UK): Witherby; 1925.
77. Smith
HI. Ethnobotany of the Gitksan Indians of British Columbia. Ottawa (ON): Canadian Museum of Civilization; 1997.
Canadian Ethnology Service Mercury Series Paper 132.
78. Turner,
NJ. Plants of Haida Gwaii. xàadlaa gwaay
guud gina k’aws (Skidegate), xàadlaa gwaayee guud ginn k’aws
(Massett). Winlaw, B.C.: Sono Nis Press, In Press.
79. Turner
NJ. The ethnobotany of the Bella Coola Indians of British Columbia. Syesis 1973:6:193-220.
80. McGregor
M. Native medicine in Southeast Alaska: Tsimshian, Tlingit, Haida. Alaska
Med 1981;23(6):65-9.
81. Deagle
G. Haida medicine, unpublished notes. Prince George (B.C.): 1985.
82. Galloway
B. Upper Stó:lo ethnobotany, Stó:lo Sitel Curriculum. Sardis (B.C.): Coqualeetza Education Training Centre;
1979.
83. Compton
BD. Upper North Wakashan and Southern Tsimshian ethnobotany: the knowledge
and usage of plants and fungi among the Oweekeno, Hanaksiala (Kitlope and
Kemano), Haisla (Kitamaat) and Kitasoo Peoples of the South Central and North
Coasts of British Columbia [dissertation].
Vancouver (B.C.): University of British Columbia; 1993.
84. Gill
SJ. Ethnobotany of the Makah and Ozette People, Olympic Peninsula,
Washington (USA) [dissertation]. Pullman
(WA): Washington State University; 1983.
85. Turner
NJ, ed. Plants of the Xaxl’ep (Stl’atl’imx, or Fraser River Lillooet) People
of British Columbia. Victoria (B.C.):
Xaxl’ep First Nation, Lillooet First Nation and The School of Environmental
Studies, University of Victoria; 1998.
86. Gunther
E. The ethnobotany of Western Washington.
Seattle (WA): University of Washington Press; 1973.
87. Turner
NJ, Timmers J. Sechelt Plant Names.
Vancouver (B.C.): The Botanical Garden, University of British Columbia; 1972.
88. Bouchard
R. Bella Coola ethnobotany, unpublished Field Notes. Victoria (B.C.): British
Columbia Indian Language Project; 1977.
89. Davis
A. 1993. Some traditional and contemporary roles of plants amongst the
Sekani People of Northeastern B.C.
[directed studies report]. Victoria (B.C.): University of Victoria; 1993.
90. Turner
NJ. Lillooet ethnobotany (Fraser River
dialect). Vancouver (B.C.): The Botanical Garden, University of British
Columbia; 1972.
91. Graham
FK. Plant lore of an Alaskan Island.
Anchorage (AK):Alaska Northwest Publishing Company; 1985.
92. Marles
RJ, Clavelle C, Monteleone L, Tays N, Burns D. Aboriginal plant use in
Canada’s northwest boreal forest. Vancouver
(B.C.): UBC Press; 2000.
93. Steedman
EV, ed. The ethnobotany of the Thompson Indians of British Columbia [based on field notes by James A. Teit]. Bureau of
American Ethnology 30th Annual Report. Washington (D.C.): Smithsonian
Institution; 1930.
94. Birket-Smith
K. The Chugach Eskimos. Copenhagen
(Denmark): Nationalmuseet Skrifter. Etnografisk Raekke VI. Nationalmuseets
Publikationsfond; 1953.
95. Smith
H.I. Materia medica of the Bella Coola and neighbouring tribes of British
Columbia. Ottawa (ON): King’s Printer;
1928. National Museum of Canada Bulletin 56.
96. Teit
JA. The Shuswap: Memoirs of
the American Museum of Natural History, The Jesup North Pacific Expedition Vol 2, Part 7. New York (NY): G.E. Stechert and Co;
1909.
97. Turner
NJ, Hebda RJ. Contemporary use of barks for medicine by two Salishan Native
elders of Southeast Vancouver Island, Canada. J Ethnopharmacol 1989;29:59-72.
98. Thommasen
HV. Medicinal plants of the Nuxalk Natives of Bella Coola. British Columbia
Medical Journal 1995;37:403-08.
99. Bouchard
R. Sechelt ethnobotany: unpublished field notes. Victoria (B.C.): British
Columbia Indian Language Project; 1978.
100. Turner
NJ, Hebda RJ, Montler T. Some important plants of the Ts’enichlhen (Saanich)
people of Southern Vancouver Island.
Victoria (B.C.): School of Environmental Studies, University of Victoria; 2000.
101. Morice
AG. Notes archaeological, industrial, and sociological on the Western Dénés. Transactions
of the Canadian Institute 1882-1893.
102. Birket-Smith
K, de Laguna F. The Eyak Indians of the Copper river delta, Alaska. Copenhagen (Denmark): Levin and Munksgaard; 1938.
103. Emmons
GE. The Tlingit Indian. Seattle (WA):
University of Washington Press; 1991.
104. Vogel
VJ. American Indian medicine. Norman
(OK): University of Oklahoma Press; 1970.
105. Kari
PR. Dena’ena K’et’una [Tanaina plantlore].
Anchorage (AK): Adult Literacy Laboratory; 1977.
106. Garibaldi A. Medicinal
flora of the Alaska Natives.
Anchorage (AK):Alaska Natural Heritage Program and the University of Alaska
Anchorage; 1999.
107. Johnson-Gottesfeld
LM. Plants, land, and people. A study of Wet’suwet’en ethnobotany [master’s thesis]. Edmonton (AB): University of
Alberta; 1993.
108. Bouchard
R. Mainland Comox ethnobotany, unpublished field notes. Victoria, (B.C.):
British Columbia Indian Language Project; 1976.
109. Wilson
S, Pierre P, Howard M, Russell G. Some medicinal remedies of the Gitksan
people, unpublished manuscript. Kitsegukla (B.C.): Kitsegukla Band; 1984.
110. Turner
NJ, Bell MAM. The ethnobotany of the Southern Kwakiutl Indians of British
Columbia. Economic Botany
1973;27:257-310.
111. Garfield
VE, Wingert PS. The Tsimshian Indians and their arts. Seattle (WA): University of Washington Press; 1966.
112. Boas
F. The Religion of the Kwakiutl Indians.
New York (NY): Columbia University Press; 1930. Columbia University
Contributions to Anthropology 10.
113. Boas
F. Kwakiutl ethnography. Chicago (IL):
University of Chicago Press; 1966.
114. U’mista
Cultural Society, Pasco J, Compton BD. The living world: plants and animals
of the Kwakwaka’wakw. Alert
Bay (B.C.): U’mista Cultural Society; 1998.
115. Turner
NJ, Bouchard R, Kennedy DID. Ethnobotany of the Okanagan-Colville Indians of
British Columbia and Washington. Victoria
(B.C.): Royal British Columbia Museum; 1980. Brit
|