Issue: 63 Page: 38-43
The Role of Chemical Reference Standards as Analytical Tools in the Quality Assessment of Botanical Materials: A European Perspective
by Klaus Reif, Hartwig Sievers, Jens-Peter Steffen
HerbalGram. 2004; 63:38-43 American Botanical Council
The Role of Chemical Reference Standards as Analytical Tools in the Quality Assessment of Botanical Materials– A European Perspective
Summary: The
increased focus on quality control in the herbal industry and the publication
in 2003 of proposed Good Manufacturing Practices (GMPs) by the Food and Drug
Administration has generated increased interest in laboratory methods to
determine the identity, purity, quality, and potency of botanical raw materials
as well as finished botanical products. Laboratory methods include microscopic
analysis of botanical characteristics of herbs as well as analysis of the plant
material’s chemistry. Analysis of chemical constituents of herbal materials is
one of the primary areas of interest among quality control technicians. Various
chemical methods are available to detect the presence or absence of “marker”
compounds and/or active compounds in herbal extracts, as well as to detect the
presence or absence of potential adulterants. In order for various chemical
methods to function, the laboratory must have access to high quality “reference
substances,” including pure “reference standards,” the pure compounds used to
calibrate the analytical machinery. This article discusses the definition of
pure chemical reference standards and their vital role in herbal quality
control.
A discussion about reference substances should begin with
the concept of inherent quality. This notion is connoted in the phrase, “You
cannot test quality into a product. You can only produce it.” That is,
appropriate testing is a necessary tool to determine the inherent quality of a
substance.
GMPs and Reference Substances
Since the publication in March 2003 by the U.S. Food and
Drug Administration (FDA) of proposed new good manufacturing practices (GMPs)
for herbal products,1 there has been increased interest in all areas
of the herb industry regarding issues of assuring quality and proper identity
of herbal raw materials and finished products.
For many years prior to the FDA’s publication of the
proposed GMPs, responsible manufacturers of botanical products had already
committed to improved procedures to ensure quality control and quality
assurance. Many of these procedures were beyond the formal requirements
contained in existing GMPs for conventional foods, the standard which herb and
dietary supplement manufacturers have had to meet. For many companies, quality
testing has already become an integral part of most of the steps in the entire
production process for herbal preparations. This includes harvesting and
post-harvesting steps, the extraction of the herbal preparation, and finally,
the finished product itself. The crucial point in quality testing is the
question, “How are valid and reproducible results achieved?” It is the duty of
a scientist to question his or her own results. So how can one achieve the goal
of producing reliable and “true” results?
When testing parameters like length, weight, or volume, a
scientist can rely on official reference sources that are directly traceable to
a common verified standard. However, specifying and testing the quality of
herbal products is not reducible to measuring physical dimensions; assessing
herbal quality requires dealing with the inherent complexity and variability of
botanicals. From a European perspective, particularly with respect to clinical
trials, the active principle of an herbal product is the herb itself, a complex
mixture of multiple compounds. Despite the enormous scientific efforts that
have been made in past decades to determine the mechanisms of action for herbal
preparations, there are still very few herbs in which the observed therapeutic
effects can be assigned to the activity of one or even a few defined
constituents.
Thus, the preparation in
its entirety must be regarded as the active part of an herbal product. However,
insofar as botanical nomenclature is used to define a plant in commerce, the
use of common and scientific names for herbs is not as exact as the use of
chemical names for specific chemicals and conventional single-chemical drugs.
For example, everyone in the herbal field knows that the name “valerian root”
is not as precise a definition of an active substance as is the name
“acetylsalicylic acid” (aspirin). The term “valerian root” is a generally
accepted, standardized common name referring the root (more accurately, the root, rhizome and stolons)
of Valeriana officinalis L.,
in the family Valerianaceae. Furthermore, valerian root (often referred to in
Europe by its pharmacopeial name, Radix Valerianae) is a defined entity in
various pharmacopeias (e.g., the German and European pharmacopeias). These
pharmacopeias contain specific botanical descriptions of the plant part used as
well as the range of specific marker compounds, which are specified as
preconditions for the use of the herb for official drug status. However, while
“valerian root” is a precise term for a plant material, the actual chemical content of that material can vary either widely with
respect to products so named in the marketplace, or within a narrow range of
chemical variance for officially accepted material.
Therefore, despite the use of authenticated botanical
voucher specimens to help assure proper identity (per the article on botanical
reference standards in this issue of HerbalGram), modern concepts and methods relating to the quality (i.e., chemical
consistency) of herbal materials and products pertain to phytochemical markers
and fingerprint analyses. These markers are the threads that tie together
production and quality control. The World Health Organization (WHO) states that
“the purpose of having reference substances is to achieve accuracy and reproducibility
of analytical results required by pharmacopeial testing and pharmaceutical
control in general” and that “the general use of a reference substance should
be considered as an integral part of a compliance-oriented monograph or test
procedure used to demonstrate the identity, purity and content of
pharmaceutical substances and preparations.”2 In the United States,
herbal products are regulated under different types of legal provisions than
those in the European Union (EU); however, the concept of a marker-based
quality assessment has become widely accepted in both markets. In the absence
of knowing the identity of the compounds specifically responsible for
biological activity, marker compounds have been used frequently as surrogates
for quality determination.
Marker Compounds
In the EU the
marker-approach is obligatory at least for those herbal products that are
registered medicinal products. According to the EU’s Committee for Proprietary
Medicinal Products (CPMP),3 herbal medicinal products must contain
only herbal drugs or herbal drug preparations as active substances. For each
herbal drug or herbal drug preparation, a comprehensive specification
established on the basis of relevant scientific data must be submitted. The
requirements are laid down in the CPMP Note for Guidance on Specifications: Test Procedures and Acceptance Criteria for
Herbal Drugs, Herbal Drug Preparations and Herbal Medicinal Products.4 The specification must provide
details of the characteristics, identification, and purity tests.
Quantification of substances with known therapeutic activity or markers is
obligatory. When pharmacopeial monographs with defined markers are not
available, the applicant should select and identify suitable markers (“or other
justified determinations”). The choice of markers should be justified.
In the U.S., herbal products can be sold as conventional
foods (e.g., spices, teas), drugs, or dietary supplements. The regulations are
set down in the Federal Food, Drug and Cosmetic Act (FDCA), with the provisions
laying the groundwork for dietary supplement regulation provided in the
amendment to the FDCA, which is called the Dietary Supplement Health and
Education Act of 1994 (DSHEA). The product category depends on intended use of
the product. A few herbs are still approved as non-prescription,
over-the-counter (OTC) drugs, e.g., the stimulant laxative senna (Senna
alexandrina Mill., Fabaceae; syn. Cassia
senna L.) and the bulk laxative psyllium
seed (Plantago arenaria Waldst. & Kit., Plantaginaceae.) Under the provisions of the FDCA, package labels are
required to accurately reflect the contents of the package. There is no
regulation or legal requirement that herbal dietary supplements or
extracts used in dietary supplements should
be characterized by declaring the content of marker compounds on the label.
According to FDA’s recently published guidance document for
Botanical Drug Products,5 label declarations of marker compound
content are not required for a botanical
preparation that is intended to be evaluated for approval as a drug. FDA has written, “In the initial stage of clinical
studies of a botanical drug, it is generally not necessary to identify the
active constituents or other biological markers or to have a chemical
identification and assay for a particular constituent or marker. Identification
by spectroscopic and/or chromatographic fingerprinting and strength by dry
weight (weight minus water or solvents) can be acceptable alternatives.” With
respect to combinations of herbs, FDA says, “When multiple active constituents
or markers are known, they should be chemically characterized and their
relative amounts should be defined.”
However, a botanical
drug product would usually have to conform to the criteria set forth in a
monograph published by the United States Pharmacopeia, which may include a
marker or group of markers and/or a fingerprint as a standard. Currently,
almost all of the monographs published in the Dietary Supplement Section of the
USP have incorporated within
them an assay for a marker or markers. If a botanical product were approved as
a drug by the FDA, the USP would likely move the monograph from the Dietary
Supplement section to the United States Pharmacopeia.
In the EU many phytomedicinal products are produced in
accordance to standards that are established to allow the product to be
licensed as a drug. Accordingly, there is currently a significant tendency by
European phytomedicine manufacturers to use a variety of phytochemical marker
substances for the characterization of a particular herbal material. In the
U.S., where herbal dietary supplements are often sold as dry powders of the raw
material or as tinctures, such characterization is not required, although the
identification of various marker compounds and the use of chemical reference
standards in validated analytical methods can provide an additional measure to
help ensure quality of the botanical material.
Many botanicals manufacturers and publishers of botanical
monographs (e.g., the USP, European Pharmocopoeia [EP]) have set standards for minimum content of certain natural
phytochemicals in raw materials and finished products. When these compounds
have known therapeutic activity, assuring minimum content can assure
therapeutic quality of the material. Unfortunately, the constituent or
constituents responsible for the therapeutic activity of most botanicals is
still unknown. In many of these cases, one or more natural constituents have
been selected by manufacturers or monograph writers as surrogates for
determination of quality of materials. These surrogate quality indicators are
referred to as marker compounds. Generally, from a European phytomedicinal
perspective, marker compounds should exhibit the following characteristics:
• be characteristic or unique for the herbal material or herbal preparation;
• be a substance with an established chemical structure;
• be present in the herbal drug preparation and finished product in sufficient
amount to allow development and creation of a scientifically valid analytical
method for both;
• be accessible to quantification with common analytical equipment (e.g., gas
chromatography [GC], high-performance liquid chromatography [HPLC], high-performance
thin-layer chromatography [HPTLC]) to keep costs of routine analysis moderate;
• be sufficiently stable under the conditions of storage and use of the final
product;
• not be present in other herbs contained in the finished product when it is
necessary to selectively quantify the content of one herb or herbal preparation
in a multi-component product; and
• be commercially available or able to be isolated by the company in its own
laboratory.
Table 1 shows some popular herbs and their typical marker substances. The marker
substances can be designated as such based on their use of quality control purposes
and/or because they are known active compounds.
Table 1: Selected popular herbs and their typical marker substances
| Herb Common Name (Latin binomial) [Compendial Reference]* |
Standard(s) |
Category† |
| Asian Ginseng (Panax ginseng) NF21 |
Ginsenosides Rg1/Rb1 |
M |
| Black Cohosh (Actaea racemosa, syn. Cimicifuga racemosa) |
27-Deoxyactein |
M |
| Butcher´s Broom (Ruscus aculeatus) |
Ruscogenin |
M |
| Butterbur (Petasites hybridus) |
Petasin |
M |
Devil´s Claw (Harpagophytum procumbens)
|
Harpagoside, Harpagide, 8-p-Coumaroylharpagide |
M
M |
| Ginkgo (Ginkgo biloba) NF21 |
Bilobalide, Ginkgolides A/B/C/J |
M |
| Kava Kava (Piper methysticum) |
Kavalactones |
AS, M |
| Milk Thistle (Silybum marianum) NF21 |
Silybin/Silydianin |
AS, M |
| St. John’s wort (Hypericum perforatum) NF21 |
Hyperforin, Hypericin |
M |
| Various Chinese herbs‡ (Aristolochia manshuriensis, A.
westlandii) |
Aristolochic acid |
FM |
* NF21 = National Formulary 21st ed. (published by the United States
Pharmacopeia)
† AS = proven active substance; M = marker substance; FM = foreign toxic compound
[Note: Some compounds have proven activity and can also be used as markers
due to their relative rarity or uniqueness.]
‡ Various Chinese herbs have been adulterated and/or substituted with herbs
of the genus Aristolochia, containing the nephrotoxic, carcinogenic compound aristolochic
acid. Such herbs that have been sometimes adulterated include Clematis armandii
and Stephania tetrandra, but these herbs do not contain aristolochic acid.
Herbal Reference Substances
Herbal reference
standards can be produced in two ways: (1) by isolation from natural sources
from the plant material and (2) by partial or total synthesis, i.e.,
artificially via chemical processes. Generally speaking, any compound that can
be separated from its natural matrix with sufficient chromatographic resolution
on an analytical scale can be isolated from the same source in sufficient
quantity to be useful as a reference standard. But the low concentration of
many substances of interest in their preferred herbal source may make isolation
prohibitively expensive and thus not economically viable. In some cases,
though, a higher content of the same compound may be found in herbal sources
other than the herb of interest.
In principle, chemical synthesis is an alternative. But many
herbal compounds have very complex structures and a high specificity with
regard to possible stereochemical isomers, which may make synthesis complicated
and costly. Therefore, despite the enormous progress in synthetic chemistry,
synthesis is—for economic reasons—still restricted to very few cases as long as
a given compound is needed for analytical purposes only. Recent examples for
synthetically derived reference standards are rubiadin and lucidin for noni
juice (Morinda citrifolia L.,
Rubiaceae). These toxic anthraquinones occur in parts of the noni plant, but
not in the fruits, and are therefore used to check the quality of the production
processes and the finished product. They are easily accessible by synthetic
pathways, though isolation is difficult because of the small quantity appearing
in natural sources. Also, for one of the widely known reference standards
hypericin from Hypericum perforatum
L. (see Table 1), synthesis is a good alternative to isolation.
Viewing reference substances as a basis of ensuring the quality of analytical
measurements (and by proxy, quality of the herbal material), it is evident that
the quality of the reference standards themselves is of crucial importance to
ensure a reliable and documented level of quality, safety, and efficacy of herbal
raw materials and products. Although many herbal substances are commercially available
today, the commercial analytical products are not always made from high quality
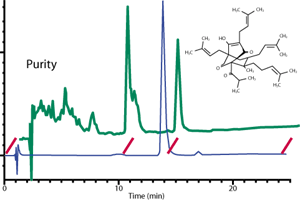
materials. For example, Figure 1 shows two commercially available batches of the
“reference standard” hyperforin (the phloroglucinol marker compound from St. John’s
wort)—both labeled for a content (by HPLC) of greater than 90%—as received in
the authors’ laboratory. By comparing the HPLC chromatograms of both, it is obvious
that only one batch (blue line) is sufficiently pure to serve as a reference for
the quantification of hyperforin in an herbal preparation. The second batch (green
line) is useless since its labeled content is not its true content, as evidenced
by the presence of numerous additional peaks in the chromatogram. The reader will
notice that the putative hyperforin peak is not even the largest among them. It
is possible that an inadequate analytical method was used to establish purity
or that the batch was not tested regularly to assure that it did not decompose
over time.
When it comes to an
appropriate handling and use of chemical reference standards, it is necessary
to have an appreciation of the different substance types that are commonly
available. This appreciation is necessary for both economic reasons and to
ensure that the reference standard is of sufficient quality to meet its
intended use. In short, some testing processes or procedures may not require a
material that is 99.9% pure, while others might. It bears mention that purity
and costs of reference standards are directly related: the more pure the
material, the higher the cost. So using a more pure standard when a less pure
material might do is not an economically sound decision. In contrast, using a
less pure material when a more pure compound is appropriate could invalidate an
entire series of expensive analyses.
According to the WHO, there are two different types of chemical
reference substances that can be distinguished in connection with the quantification of compounds in an herbal product: primary
and secondary reference
substances.2
“A designated primary
chemical reference substance is
one that is widely acknowledged to have the appropriate qualities within a
specified context, and whose value is accepted without requiring comparison to
another chemical substance.”2 This means that only highly purified
compounds, with defined and documented identity, purity, and content can be
used as primary reference substances. Essentially, the primary reference
substance establishes a fundamental value for a specified analytical method, on
which all other definitions of content depend.
WHO states that “a
secondary chemical reference substance is a substance whose characteristics are assigned and/or calibrated by
comparison with a primary chemical reference substance.”2 This
comparison allows for the definition of the content of a particular substance
without generating the complete data about identity, purity, and content for
the substance itself. This is only possible when a primary reference substance
is available for calibration. In comparison to the field of measuring physical
dimensions, one ounce (avoirdupois) can only be defined as 0.0283 of a kilogram
when the mass of a kilogram is itself defined. Secondary reference substances
are often called working standards—a term that indicates their importance in “everyday” laboratory work.
The USP and the EP offer primary reference substances for
use with methods specified in their respective monographs. From a regulatory
point of view, despite their high purity, these pharmacopeial standards are not
valid for analytical purposes, other than their use in methods described in the
respective monographs.
Finally, any other chemical substance with known structure
can be used as a standard for identity testing, although its content may not be defined. For example, in fingerprint
analysis by thin layer chromatography (TLC) the comparison of a preparation
with marker substances shows whether or not the desired compounds are present.
Defining a Chemical Reference Substance
At this point it is necessary to discuss the determination
of the content of a reference substance. The knowledge about how the content of
a given reference substance is established
is an essential prerequisite for its appropriate use in quantitative
analyses. Unfortunately, many users of reference standards are not aware of the
danger of using a reference substance—the content of which is not clearly defined—for the quantification of a component.
The first step in
defining a chemical reference substance is always its identification. Today, nuclear magnetic resonance spectroscopy
(NMR) and mass spectrometry (MS) are the most important tools for structure
elucidation (the determination of the actual three-dimensional chemical
structure of a specific molecule). The second step is to examine its purity. Again, this requires a combination of different
methods. High performance liquid chromatography (HPLC) or gas chromatography
(GC) are typical techniques used to search for organic impurities, as is thin
layer chromatography (TLC). The content of water and residual solvents needs to
be established, and elemental analysis would reveal whether or not there are
inorganic impurities present that have to be considered. In particular cases,
purity tests may include other methods, such as optical rotation or melting
point. Then, as the third step, the content is typically determined using HPLC or GC methods.
However, the reference substance user should be aware that a test for content
based on a single HPLC or GC method is not sufficient because these analytical
methods do not provide for an absolute content value, i.e., detect all possible
organic impurities. To overcome this problem it is widely accepted to
establish the content based on at least two independent methods to ensure that
the assigned value is the “true” one. Until the total amount of impurities is
subtracted from this value, it is not possible to speak about the defined
content of a reference standard
(e.g., for primary reference substances).
Because of these complex,
time-consuming measurements, “the production, validation, maintenance and
distribution of chemical reference substances is a costly and time-consuming
undertaking.”2 For the user of reference substances this statement
simply means that reference standards, especially verified reference standards,
are expensive materials and it is of high importance to optimize their use.
In most day-to-day lab work, the ideal procedure for
quantification of a single compound in a mixture—comparison with a verified reference
standard—is impractical due to the high costs of the standards. Therefore, the
most common procedure is to buy a small amount of a commercially available
primary reference substance, or generate one’s own primary substance, and to
calibrate the content of cheaper bulk batches (i.e., less pure) of the same
substance by comparison with the primary substance, thus producing secondary or
working standards. This process provides a defined content for the working standard. The approach saves
money because the amount of primary reference substance needed for comparison
is lower and because only a single analytical method is generally required to
assign the content value to the secondary standard.
But some pitfalls must be considered carefully. Usually, reference
substances are used over a long period of time; for example, in routine
analysis or stability testing. But the content of a reference substance depends
on variable factors:
• Decomposition may occur, especially if the substance is not properly protected
against oxygen and/or light.
• The water content and the content of other residual solvents may change if
the reference substance is not stored in an airtight vessel.
• Consequently, storage of reference substances under defined conditions in
a cool, dry, dark place is essential, but it does not eliminate the need to retest
regularly.
However, sooner or later a batch will be completely
depleted. In view of this inevitability, every new batch of a reference
substance should be tested by direct comparison with the preceding batch.
Further, it is important to order new reference material before the previous
batch is exhausted. In the authors’ opinion, this is the only way to provide comparability
and traceability of content values in the long-term.
Most often, users are
left to discover all these requirements concerning the methodically correct use
of reference substances on their own. In the authors’ opinions, it should be
possible for users to choose whether they want to perform all the procedures
described above on their own, or whether they will simply purchase a working
standard from a reputable supplier who offers long-term traceability of the
calculated content values.
A supplier of reference substances could offer (1) “primary”
or verified reference substances with a defined content and complete
documentation available, and (2) ready-to-use working standards for which the
calibration against the “primary” substance is clearly and traceably
documented.
By providing such documentation the requirements regarding
(1) stability testing, (2) examination for impurities, (3) definition of
storage conditions, and (4) documentation of all these procedures are minimized
for users. Consequently, users finally get what they really need: reference
substances, characterized through a defined content, at reasonable cost, and
traceable to a basic “primary” value for a sufficient period of time. In this
way, manufacturers of herbal products can help to ensure that the analytical
testing—which will probably be required for many herbal products under new GMP
regulations—will be based on high-quality reference substances. In the opinion
of these authors, this will ultimately result in the production of more herb
products of reliable quality.
Klaus Reif has a doctorate in Chemistry. A widely
acknowledged expert in the field of analytical chemistry, he is the Head of the
Department of Instrumental Analysis at PhytoLab in Germany, a large service
laboratory for herbal products and a leading European supplier of chemical
reference standards for botanicals. A member of the AOAC´s Pool of Experts, he
belongs to several Expert Review Panels of the AOAC for Dietary Supplements. He
has publications on various analytical subjects, e.g., in the Journal of Chromatography.
Hartwig Sievers has a doctorate in Pharmacy
(Pharmacognosy) with extensive experience in regulatory affairs and quality
control of herbal medicinal products (HMPs). He is the Head of the Department
of Pharmaceutical Services and Research and Development at PhytoLab. His
present focus is the safety and efficacy, pharmacovigilance, and development of
new HMPs. He has publications in Phytochemistry,
Clinical Pharmacology and Therapeutics, and Herba Polonica.
Jens-Peter Steffen has a doctorate in Chemistry specializing
in synthetic organic chemistry and natural products research. He is Technical
Manager of the PhytoLab Reference Standards Department. He has published papers
on natural products chemistry in Phytochemistry
and the Journal of Agricultural and Food Chemistry.
References:
1. Current Good Manufacturing Practice in Manufacturing,
Packing or Holding Dietary Ingredients and Dietary Supplements: Proposed Rule. Federal
Register Vol. 68, No. 49, Docket No.
96N-0417. Washington, DC: Food and Drug Administration. March 13,
2003;12158-12263.
2. General guidelines for the establishment, maintenance and distribution of
chemical reference substances, WHO Technical Report Series, No. 885, 1999 (Annex
3). Available at http://www.who.int/medicines/organization/qsm/activities/qualityassurance/pharmacopea/who-trs-885annex3.html.
3. Note for Guidance on quality of herbal medicinal products, Committee for
Proprietary Medicinal Products (EU) /QWP/2819/00. Available at http://www.emea.eu.int/index/indexh1.htm
(Homepage of the EMEA Herbal Medicinal Products Working Party [HMPWP]).
4. Note for Guidance on specifications: test procedures and acceptance criteria
for herbal drugs, herbal drug preparations and herbal medicinal products, Committee
for Proprietary Medicinal Products (EU) /QWP/2820/00. Available at http://www.emea.eu.int/index/indexh1.htm
(Homepage of the EMEA Herbal Medicinal Products Working Party [HMPWP]).
5. U.S. Department of Health and Human Services Food and Drug Administration
Center for Drug Evaluation and Research (CDER), Guidance for Industry: Botanical
Drug Products. Jun, 2004. Available at http://www.fda.gov/cder/guidance/index.htm.
Reference Standards
Reference standards are highly purified compounds used in
the testing of various materials, whether they are herbal or conventional
drugs. A small amount of a reference standard is used as the basis for the
detection of the presence and quantity of the same compound in the material
being tested. Examples of reference standards include specific ginsenosides
(e.g., Rg1, Rb1) from Asian ginseng (Panax ginseng C.A. Meyer, Araliaceae) or American ginseng (P.
quinquefolius L.), the terpenes ginkgolide
A, B and C or bilobalide in ginkgo (Ginkgo biloba L., Ginkgoaceae), hypericin or hyperforin in St.
John’s wort (Hypericum perforatum L.,
Clusiaceae), etc. Theoretically, pure reference standards can be developed by
extraction and isolation in an herb company’s own laboratory, but the
technology and the costs to do this may be prohibitively high. Most analytical
laboratories obtain reference standards from (1) chemical supply companies that
specialize in the sales of laboratory chemicals, including reference standards,
reagents, and other specialized chemicals or (2) directly from the few
companies that specialize in the production and sale of reference standards for
herbal products. The purity of these compounds can vary unless they are
produced according to exacting chemical standards.
|