Issue: 66 Page: 35-48
A Critical Review of Herbal Remedies for Poison Ivy Dermatitis
by David S. Senchina
HerbalGram. 2005; 66:35-48 American Botanical Council
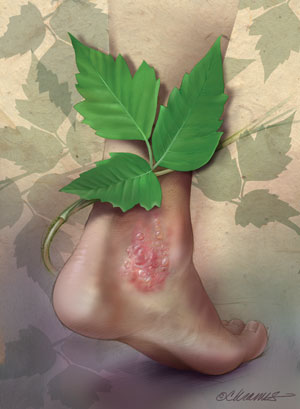 |
Illustration by Christy Krames, MA, CMI
Christy Krames is a Certified Medical Illustrator living and working in Austin,
Texas. She received her Master's degree in Medical Illustration in 1981 from
UT Southwestern Medical Center in Dallas. Examples of her medical and biological
artwork may beseen at www.kramestudios.com. |
Poison ivy is well known for the painful, sometimes
long-lasting lesions it may afflict on sensitive individuals. The plant, a
member of the family Anacardiaceae, is known by several Latin binomials in the
scientific and botanical literature: Toxicodendron radicans (L.) Kuntze, T. rydbergii (Small ex Rydb.) Greene, and Rhus radicans (L.). A bewildering number of herbal remedies, which
vary widely in efficacy, are suggested by both the popular and scientific
literature for treating poison ivy dermatitis (hereafter Toxicodendron dermatitis,
TD).
This critical review summarizes the existing medical data
relating to the capability of these plants to heal inflammatory skin disorders
such as TD. Due largely to a lack of research, many remedies have scientifically
unproven efficacy, such as gumweed (Grindelia spp. Willd., Asteraceae). Other recommended remedies have
scientifically disproven efficacy. An especially poignant example is jewelweed
(Impatiens capensis Meerb. and I.
pallida Nutt., Balsaminaceae), perhaps the
most popular traditional herbal remedy for treating TD, which has been
discredited by a number of studies.
Though these findings may at first seem disheartening, there
are several herbal remedies that have
demonstrated efficacy in treating inflammatory skin conditions similar to TD.
Among this category are echinacea, aka purple coneflower (Echinacea spp. Moench, Asteraceae) and witch hazel (Hamamelis
virginiana L., Hamamelidaceae). Continuing
research in the field will likely expand this list in upcoming years.
Poison Ivy and Skin Rashes
In a previous issue of HerbalGram, Armstrong and Epstein1 discussed the
history, biology, chemistry, and toxicity of the genus Toxicodendron to which poison ivy belongs, and they addressed some
popular remedies for both treatment and prevention of lesions. Some major
points that are immediately pertinent to this discussion are reviewed below.
Two species of poison ivy are recognized from Northern
America: eastern poison ivy (T. radicans)
and its sister species, western poison ivy (T. rydbergii). Depending on the botanist, several subspecies of
eastern poison ivy may be acknowledged.2 This genus also harbors a
number of other poisonous plants, including Pacific poison oak (T.
diversilobum [Torr. & Gray] Greene),
Atlantic poison oak (T. pubescens
P. Mill.), and poison sumac (T. vernix [L.] Kuntze). Exposure and reaction to any one of these plants will
render an individual reactive to all others due to allergen similarity.3
For ease of reference, the dermatitides caused by all of these plants will be
considered under TD.
Poison ivy’s powerful allergens, known collectively
as urushiol, easily transfer to human skin. Urushiols are alkenyl polyphenols.4
To spark an immune response, these relatively small chemicals must first bind
to proteins on skin cells5 which then sets off a chain reaction
involving many aspects of the immune system.6 Typically, this series
of events takes two to several days to culminate, and people often don’t
realize they’ve contacted the plant until the hypersensitivity reaction is well
underway. Additionally, poison ivy often goes unrecognized in the field either
because people aren’t looking for it or don’t recognize it (poison ivy exhibits
a high degree of morphological variability contingent on genetic and
environmental factors).6 A list of plants containing urushiol and
producing TD and related inflammations is shown in Table 1.
| Table 1. Selected plants containing the contact
allergen urushiol which can produce TD and related dermatitides. |
| Scientific Name |
Family |
Common Name(s) |
| Anacardium occidentale L. |
Anacardiaceae |
Cashew (nut shell) |
| Ginkgo biloba L. |
Ginkgoaceae |
Ginkgo, Maidenhair tree |
| Lithraea spp. Endl. |
Anacardiaceae |
|
| Mangifera indica L. |
Anacardiaceae |
Mango |
| Metopium toxiferum Krug. |
Anacardiaceae |
Poison wood |
| Schinopsis spp. Engler |
Anacardiaceae |
Quebracho, schinopsis |
| Schinus spp. L. |
Anacardiaceae |
Peppertree |
| Semecarpus spp. L. |
Anacardiaceae |
Marking nut tree, cashew |
| Smodingium argutum E. Mey. |
Anacardiaceae |
Rainbow leaf |
| Swintonia floribunda Griff. |
Anacardiaceae |
Rengas |
| Toxicodendron diversilobum (Torr. & Gray) Greene |
Anacardiaceae |
Pacific poison oak |
| Toxicodendron pubescens P. Mill. |
Anacardiaceae |
Atlantic poison oak |
| Toxicodendron radicans (L.) Kuntze |
Anacardiaceae |
Eastern poison ivy |
| Toxicodendron rydbergii (Small ex Rydb.) Greene |
Anacardiaceae |
Western poison ivy |
| Toxicodendron vernicifluum (Stokes) F. Barkley |
Anacardiaceae |
Lacquer tree |
| Toxicodendron vernix (L.) Kuntze |
Anacardiaceae |
Poison sumac |
The cardinal sign of TD is inflammation, which has four main
components: heat, pain, erythema (redness), and edema (swelling). Vesiculation
(blistering) can also occur and, when severe, there is weeping of serous fluid
from the affected sites. The primary symptom is pruritis (itching), which is
usually episodically intense, followed by a refractory (regressive) period.
Pain and tenderness may be present on occasion where there is intense, tight
edema.
Herbal remedies that effectively alleviate the symptoms of
TD frequently target one or more of these components, either by acting directly
on the immune system itself, or indirectly by acting on products of the immune
system. Still others provide analgesic effects.
Diversity of Herbal Remedies for Toxicodendron Dermatitis
A catalogue of over 175 different herbal remedies that
have been utilized in the treatment of TD has been compiled during the research
to prepare this article. Twenty-five of the most frequently-mentioned remedies
have been distilled from this larger set and are presented in Table 2. As is
apparent from the Table, herbal remedies for poison ivy dermatitis are
phylogenetically diverse, coming from both “primitive” and “recent” plant
lineages. Ferns, grasses, herbs, shrubs, and trees have all been utilized.
| Table 2. Most frequently mentioned herbal remedies
for TD as determined through a survey of over 300 print and Internet resources. |
| Scientific Name |
Family |
Common Name(s) |
Scientific Verification* |
| Aloe vera (L.) Burm. f. |
Aloeaceae |
Aloe |
Suggested |
Arctium lappa L.,
Arctium minus Bernh. |
Asteraceae |
Burdock |
Unproved |
Artemisia vulgaris L. |
Asteraceae |
Mugwort |
Unproved |
| Calendula officinalis L. |
Asteraceae |
Calendula, Marigold |
Suggested |
| Commiphora spp. Jacq. |
Burseraceae |
Myrrh, Balm |
Suggested |
| Comptonia peregrine (L.) Coult. |
Myricaceae |
Sweet Fern |
Unproved |
| Echinacea spp. Moench |
Asteraceae |
Coneflower, Echinacea |
Suggested |
| Grindelia spp. Willd. |
Asteraceae |
Grindelia, Gumweed |
Unproved |
| Hamamelis virginiana L. |
Hamamelidaceae |
Witch hazel |
Suggested |
| Hydrastis canadensis L. |
Ranunculaceae |
Goldenseal |
Unproved |
Impatiens capensis Meerb.,
I. pallida Nutt. |
Balsaminaceae |
Jewelweed,Touch-me-not |
Disproved |
| Linum spp. L. |
Linaceae |
Flax |
Unproved |
| Lobelia spp. L. |
Campanulaceae |
Lobelia |
Suggested |
| Matricaria recutita L. |
Asteraceae |
Chamomile, Mayweed |
Suggested |
| Melaleuca spp. L. |
Myrtaceae |
Melaleuca, Tea Tree |
Suggested |
| Mentha X piperita L. (pro sp.) |
Lamiaceae |
Peppermint |
Suggested |
| Plantago spp. L. |
Plantaginaceae |
Plantain, Indianwheat |
Suggested |
| Quercus alba L. |
Fagaceae |
White Oak |
Unproved |
| Rumex spp. L. |
Polygonaceae |
Dock |
Suggested |
| Sassafras albidum (Nutt.) Nees |
Lauraceae |
Sassafras |
Unproved |
| Symphytum officinale L. |
Boraginaceae |
Comfrey |
Unproved |
Toxicodendron radicans (L.) Kuntze,
T. rydbergii (Small ex. Rydb.) Greene |
Anacardiaceae |
Poison Ivy |
Unproved |
| Urtica dioica L. |
Urticaceae |
Nettle |
Suggested |
| Verbascum thapsus L. |
Scrophulariaceae |
Mullein, Flannel Plant, Velvet Dock |
Suggested |
Note: For the inclusion of an herbal remedy in this table,
tallies were made when (a) a source specifically mentioned the plant’s
use in treating TD, or (b) commercial products designed specifically for TD used
the plant as one of their ingredients.
* Scientific studies examining a plant’s utility in this capacity were
excluded in tallying frequency counts, but they were the sole component considered
in the “scientific verification” column. In this category, “unproved” denotes
a plant for which no controlled, scientific studies exist documenting any activities
(such as anti-inflammatory activity) that may be associated with TD treatment. “Suggested” indicates
a plant for which (a) at least one scientific study has demonstrated that the
plant has some activity that may be associated with TD treatment, but (b) no
studies specifically examining the plant’s effects on TD treatment exist. “Disproved” indicates
a plant for which scientific studies have found the plant ineffectual in TD treatment
specifically—at least within the context of the specific preparation and
mode of administration utilized in the cited study. The reader should note whether
a genus or species is being considered for each entry. |
Fourteen of these plants
are discussed in detail below and are divided into three groups based on
indications and popularity: (1) plants used frequently and specifically for TD;
(2) plants indicated for skin disorders sensu lato (in the broad sense); and (3) other plants
of merit.
Plants Used Frequently and Specifically for Toxicodendron
Dermatitis
Gumweed
Gumweed (Grindelia
spp. Willd., Asteraceae) flowering tops and leaves are frequently recommended
by the herbal literature for treating TD. Its popularity stems from a long
history of usage beginning with Native Americans, who employed it specifically
for this condition (and others).7, 8 In the mid-nineteenth century,
the efforts of Dr. C. A. Canfield helped catapult gumweed into popular use
among Anglo-American populations,8 where it has since been
perpetuated.
Aqueous extracts or infusions are typically employed.
Writing in the early 1900s, Sollmann recommended that a diluted fluid extract
be used to wash the afflicted site.9 In 1936, the American
Pharmaceutical Association suggested that the fluid extract should be combined
with sodium bicarbonate, sodium sulfate, glycerin, and water to make a poison
ivy lotion once known as Patton’s lotion.10 Alternatively, bark
infusions of gumweed, lobelia herb (Lobelia
spp. L., Campanulaceae), or sassafras (Sassafras albidum
[Nutt.] Nees, Lauraceae) may be utilized topically.11 Gumweed is a
frequent ingredient of commercial herbal products designed to treat TD.
To date, few medical studies of this plant have been
reported, preventing any recommendations on its usage in the context of TD.
However, no reports of adverse reactions to topical preparations have been
reported, suggesting that topical use of gumweed is relatively safe. Phenols in
the plant may be responsible for its anti-inflammatory properties.8
Jewelweed
Unquestionably, jewelweed (Impatiens capensis Meerb., Balsaminaceae; I. pallida Nutt.,
Balsaminaceae) is more frequently cited for treating TD than any other herbal
remedy. Several factors quickly explain jewelweed’s popularity: (1) its widespread distribution throughout much of the
U.S.; (2) its weedy habit, often growing in the same locations as poison ivy;
(3) ease of identification; (4) the fact that both species found in the U.S.
may be used; (5) its convenience—no preparation is necessary; and (6) the
absence of adverse reaction reports when it is used in this manner.
Various groups of Native Americans employed jewelweed for
numerous ailments,7 especially skin disorders such as TD. 7, 12
Most often the succulent stems were crushed and their juices applied directly
to the lesions. The same mode of treatment is commonly used today.
Additionally, jewelweed has become the premier ingredient of many commercial
herbal remedies for TD that usually employ a combination of several herbs.
Jewelweed’s fame has attracted much scientific scrutiny.
While it is supported by an overwhelming number of testimonials and anecdotal
evidence, most scientific studies have found jewelweed to be ineffective in
treating TD.13-16 One early study did show support for the plant,17
and another found evidence that compounds inside jewelweed could neutralize Toxicodendron allergens under highly controlled (i.e., not
clinical) conditions.13 Compounds isolated from the corolla of a
related species, I. balsamina L.,
have shown selective cyclooxygenase-2 inhibiting (anti-inflammatory)
properties.18 Despite these unfavorable results, jewelweed is still
highly acclaimed and firmly embedded in herbal lore, buttressed by a large
number of testimonials proclaiming jewelweed’s efficacy from credible sources.19-23
No adverse reactions to jewelweed have been reported in the scientific
literature.
Plantain
Plantain (Plantago
spp., Plantaginaceae) is a popular remedy for many of the same reasons as
jewelweed. Native Americans employed numerous members of the genus for healing
skin disorders (frequently burns) and as analgesics.7, 12 Both
traditional Native American and more recent Anglo-American practices employ
above-ground parts of plantain topically.24 Raw leaves are crushed
and rubbed over affected areas. Less frequently, tinctures, extracts, or
infusions are made; several modern commercial preparations contain plantain.
Little has been written about plantain as a cure for TD in
the scientific literature, but it has been mentioned specifically for this
purpose.22, 25 Both P. lanceolata and P. major have been
noted for their anti-inflammatory qualities by several sources23, 26, 27
including Culpepper, an English herbalist of the 1600s who praised P.
major for its ability to “hinder
inflammations.”28 Duckett, in a letter to the editor of The
New England Journal of Medicine, described
an impromptu experiment where the leaves of P. lanceolata were used to successfully ameliorate TD in a group
of 10 people.25 In laboratory studies, P. lanceolata has demonstrated anti-inflammatory activity,29
and P. major has demonstrated
anti-nociceptive (pain-killing) properties.30 This research suggests
plantain may be effective in the treatment of TD, but further studies are
needed. There are no reports of adverse reactions to this herbal remedy.
Poison Ivy
Toxicodendron species (e.g., T. radicans, T. rydbergii) themselves have long been esteemed for both
prevention and treatment of the lesions they inflict,31-35 alongside
other skin disorders. Native Americans would chew fresh young leaves of the
plant (generally those emerging in the springtime) to prevent TD from
occurring,7 and this practice was transmitted to Anglo-Americans.36,
37 Less frequently, tinctures were made from fresh plant or dried
roots and taken orally to achieve the same effect.7, 33 The goal in
all cases was to induce hyposensitization in susceptible individuals so that
their bodies would ignore the inflammatory effects of urushiol upon future
exposure.
While hyposensitization was regularly practiced by
some groups of Native Americans for centuries, it didn’t appear in Western medical
journals until the mid 1800s where it was reported with some caution.38
Kligman36, 39 aptly reviewed the history of poison ivy
hyposensitization up to 1960 and concluded that temporary hyposensitization was
plausible via either intramuscular or oral administration of poison ivy
extract. However, he also emphasized that while much anecdotal evidence
supported these claims, there was little science to substantiate them.
Controlled experiments addressing poison ivy
hyposensitization have since been conducted. Scientists first demonstrated that
this technique was feasible in guinea pigs before demonstrating efficacy in
humans.40-43 Importantly, these studies differed in their mode of
administration and population specifics, which explain variation among the
results. No studies have yet been conducted to explore preventative effects
from chewing Toxicodendron leaves.
In addition to inducing hyposensitization, Native Americans
also used Toxicodendron species to treat
poison ivy reactions already underway. Most frequently, fresh leaves were
rubbed over affected areas to promote healing.7 Several
Anglo-American authors stated that drying the leaves resulted in a loss of
medicinal activity.32, 44 However, urushiol remains dangerous years
after herbarium specimens of poison ivy are prepared.45 The fruit
juice and seeds of Toxicodendron
species were also used topically by Native Americans to heal other skin wounds.34
While no statements can be made concerning the use of Toxicodendron as a treatment for TD due to an absence of research,
several conclusions may be reached concerning Toxicodendron hyposensitization. First, determining the
appropriate dosage is exceedingly difficult, typically requiring large amounts
of urushiol over long time periods. Second, adverse effects are frequent when
using this remedy. Individuals taking Toxicodendron preparations for conditions other than TD have also
reported negative consequences.46-48 Third, any protection obtained
via this route is only transient. Most studies showed persistence of tolerance
for only a short period of time following withdrawal of the hyposensitizing
stimulus. Fourth, products of this plant vary wildly in both content and
efficacy depending on the mode of preparation. Fifth, poison ivy preparations
taken systemically will affect multiple body systems, not just inflamed areas
of skin.
All of these concerns are compounded with the practice of
leaf-chewing, where personal characteristics of both the plant and recipient
make dosing difficult. Following ingestion, dermatitis may erupt in the oral as
well as anal regions, leading to discomfort and pain.38, 49
One quickly appreciates the dangers associated with this
remedy. Individuals may avoid commercial products derived from poison ivy due
to either previous or potential side effects. Some readers may have received
poison ivy vaccines (poison ivy leaf extracts injected intramuscularly or
subcutaneously),44,50 but these have been discarded due to their
highly variable and frequently poor efficacy rate over time.
Finally, there are many
homeopathic poison ivy remedies (often sold as “Rhus tox”) in which a
homeopathic dilution of a fresh poison ivy extract is used as the primary
ingredient (homeopathic remedies from other, non-Toxicodendron sources,
are also commonly employed). These products are used both as a preventative prior
to exposure and as a treatment post-exposure. [Editor’s Note: Being highly diluted
and thus virtually free of any active ingredients (from a conventional pharmacological
perspective), they are considered quite safe.]
Remedies Indicated Broadly for Skin Disorders
Aloe
The succulent aloe plant (Aloe vera [L.] Burm. f., Aloeaceae), sometimes referred to by
the synonymous name A. barbadensis,
has been used since ancient times for a variety of medical purposes among
Mediterranean and Middle Eastern cultures.27, 51, 52 Unlike all
aforementioned herbal remedies, aloe is neither native nor naturalized in
America (although it is commercially cultivated in Florida and South Texas) and
consequently has no tradition of use among native peoples. Aloe has only
recently been engaged in the treatment of TD, based primarily on its purported
efficacy in healing numerous other skin disorders.
Reviews of the scientific literature on aloe have only
recently been supplied.53-56 Results from scientific studies are
contradictory. Used to heal wounds, fresh raw aloe gel and various aloe
preparations have been found to be efficacious, inactive, or even deleterious
by different researchers. (These findings may be the result of variations in
content and quality of some preparations used in the research). Nevertheless,
beneficial effects from aloe preparations have been documented via oral,
subcutaneous, and topical routes in almost a dozen mammal models, including
humans. Several studies have documented anti-inflammatory effects.57-59
Although much further research is needed, there is currently
no reason to doubt that aloe may perform similar anti-inflammatory actions when
applied to TD.22 Adverse reactions to aloe vera derivatives are very
rare, considering its widespread use, but have been reported.60, 61
Echinacea (Purple Coneflower)
Native Americans found Echinacea species (Echinacea spp., Asteraceae) to be versatile remedies for many
ailments, including skin disorders.7, 8, 12, 62, 63 Echinacea was spring-boarded into popularity among
Anglo-Americans, somewhat improbably, by the enterprising doctor A. Mayer who
marketed echinacea preparations as a blood purifier.56, 64 One
pharmacognosy textbook noted that topical applications of echinacea (either E.
angustifolia or E. purpurea) were used locally in the Midwest as a treatment for
TD.44
The roots and leaves (occasionally fresh, but most often
dried) are used most frequently in both homemade and commercial preparations,
typically involving a drying process. For skin disorders echinacea is typically
found in ointment or tea form, but it is also used in poultices, tablets, and
tinctures.24, 26, 27
Echinacea’s burgeoning popularity in the last decade has been
mirrored by an escalation in research efforts. Data on three of the genus’ nine
species (E. angustifolia DC, E.
pallida [Nutt.] Nutt., and E.
purpurea [L.] Moench) is mountainous and
has been critically evaluated in HerbalGram and other sources.24, 63, 65-70
Researchers are just beginning to investigate the other six species, of which
much less is known.71 Due to differences in species used, plant
parts used, and extraction method, echinacea preparations may exhibit
dramatically different effects when subjected to controlled experiments testing
their efficacy as anti-inflammatory medicines.
Rodent models of inflammation have been employed to assess
the anti-inflammatory effects of echinacea preparations when applied topically.69
Aqueous extracts72, 73 and acetic acid extracts74 from
the roots of E. angustifolia and hot
ethanol extracts from the roots of E. pallida and E. purpurea75 have all shown anti-inflammatory effects in these models.
Furthermore, hot ethanol extracts of E. pallida and E. purpurea roots have demonstrated significant wound healing (cicatrizing)
properties under similar conditions.75 Dried root powder from E.
purpurea also exhibited anti-inflammatory
qualities when fed to rodents,76 suggesting that compounds from
echinacea may be capable of exerting their anti-inflammatory effects whether
administered orally or topically in rodent models.
Echinacea preparations have also been assayed in vitro for
their ability to counteract biological processes linked to inflammation. Hot n-hexane extracts77 and purified caffeoyl
derivatives78 from E. angustifolia have all proven efficacious
in these experiments. Taken together, the data suggest that echinacea’s ability
to heal skin disorders may be linked to several biochemical constituents (alkamides,
alkylamides, caffeoyl derivatives, polyphenols, and polysaccharides), with the
proportions of these compounds varying by species and also by tissue.8,
72-79 Studies are just beginning to elucidate the molecular mechanisms
that underlie these properties.
Results from these studies and others suggest that echinacea
extracts containing these constituents possess anti-inflammatory qualities as
demonstrated in both in vivo and in vitro experiments. In addition, the German
Commission E has approved a preparation made from the fresh-pressed juice of E. purpurea herb (i.e., aerial parts) as a treatment for wounds.70
As echinacea has shown efficacy under these conditions, it is tempting to
speculate that echinacea may also be efficacious in healing TD, though this
remains to be borne out experimentally.
Peppermint
Although peppermint (Mentha x piperita L. [pro.
sp.], Lamiaceae) is not indigenous to North America,80 Native
Americans quickly discovered that it harbored medicinal properties similar to
its native relatives (specifically, M. arvensis L. and M. spicata L.). Infusions of peppermint, and less frequently
tinctures and tonics, were used by Native Americans principally to treat colds,
fevers, digestive disorders, and inflammatory conditions.7 Scientists
worldwide have produced much research focusing on peppermint’s usage
in these contexts and also skin disorders. Menthol is the principal component
responsible for peppermint’s medicinal properties;81, 82 aromas and
tastes associated with mints are attributable to esters such as menthyl
acetate.82 Both peppermint leaf and the oil distilled from the
leaves have well-corroborated medicinal benefits when used both internally and
externally for a variety of ailments.24, 70 Peppermint oil is used
to treat poison ivy dermatitis via creams, lotions, and soaps; less frequently,
it is used as a component in medicinal baths.83
Studies have shown that
peppermint oil has multiple beneficial effects on skin tissue, including
anti-inflammatory and analgesic properties;57,82 peppermint also has
strong antioxidant properties.84 Though never studied in the context
of TD, peppermint’s anti-inflammatory properties may lend themselves to this
cause and deserve further attention. Its use is deemed safe,24,56,70
although rarely adverse reactions are reported.82
Witch Hazel
Witch hazel (Hamamelis virginiana L., Hamamelidaceae) bark (sometimes leaves and
twigs) is a remedy known by many laypeople for its astringent,
anti-inflammatory, and wound-healing qualities,20 which were long
ago understood by Native Americans.7, 12, 63 Its anti-inflammatory
properties have been demonstrated in human volunteers;85,86 however,
depending on the mode of preparation the desired constituents may be lost.23,
87 Hamamelitannin and various proanthocyanidins may be responsible for
this anti-inflammatory activity.56, 63, 88 Recent data suggest that
witch hazel may be employable for a variety of chronic diseases and cancer.89,
90
For the treatment of poison ivy, witch hazel bark (sometimes
leave and twig) extracts, infusions, and ointments are typically made (with
alcohol added for preservation) and topically applied. Witch hazel is
well-tolerated by most people20 (though infrequently adverse
reactions are reported),91, 92 and a variety of commercial
preparations are typically accessible to the general population. However,
preparations sold in the U.S. are frequently lacking tannins (commercial
manufacturers often employ a distillation process, which does not retain the
tannins), believed to be the main medicinal component.23,26,56 The
limited evidence available suggests that witch hazel may be efficacious in
soothing and speeding recovery of skin disorders like TD, and it is a worthy
candidate for further specific research in TD herbal treatment.
Other Herbal Remedies
Several other herbal
remedies, for which much less is known, deserve mentioning and will be
discussed briefly here. Overall, these remedies are less popular than the
previous set described and consequently experience less use.
Burdock
Following the
introduction of burdock (Arctium lappa L. Asteraceae; A.
minus Bernh.) to North America by
Europeans,80 Native Americans employed burdock medicinally in many
contexts, including skin disorders and a variety of inflammatory conditions.7,
12 Many different suggestions have been made pertaining to the use of
burdock in treating TD. Some say the leaves should be crushed and rubbed
against the wound to relieve itching, similar to how one would use jewelweed or
plantain. The leaves also harbor antibacterial qualities.93 Others
report using the roots, either applying them in tincture or oil form on the
wound site or ingesting them in tea or tablet form. Burdock is also recommend
for other skin disorders such as acne, boils, or psoriasis.27, 28, 56, 94
No studies related to the curative effects of burdock have
yet been published; however, Lin et al95 have shown that burdock
extracts have a hepatoprotective effect which may be related to its antioxidant
activity, and anti-inflammatory activity of an isolated component (arctigenin)
has been experimentally demonstrated.96 Most of the scientific
literature on burdock relates to poisonings and allergic reactions,97-101
although in several cases purported burdock poisonings were later found to be
due to the accidental adulteration of some commercial supplies with belladonna
root (Atropa belladonna L., Solanceae),
thereby causing atropine poisoning.56 (Burdock root is widely
recognized for its lack of toxicity; the root is a staple vegetable in Japanese
cuisine, where it is known as gobo).
The lack of medical validation on burdock root precludes making any definite
comments about burdock in TD treatment.
Comfrey
The ability of comfrey (Symphytum officinale L., Boraginaceae) to heal skin disorders has been known
by many cultures, including the Greeks102 and some groups of Native
Americans7, 12 who used both its roots and leaves in a variety of
medical capacities. These are harvested and then used in poultices, oils,
ointments, teas, or tinctures.20, 26, 94 For treating TD, some
sources indicate that the leaves may be used raw similarly to jewelweed or
plantain. For skin wounds in general, both above- and below-ground components
are employed in poultices.56 Importantly, comfrey is used in a
plethora of commercial products indicated for TD.
A major drawback to comfrey is its hepatotoxic pyrrolizidine
alkaloids, which are found in many plant species.103 These compounds
have the potential to exert toxic effects and do not contribute to the
medicinal activities of the plant, which are instead attributed to other
compounds such as allantoin and triterpene glycosides (i.e., rosmarinic acid.)70,
104 Allantoin is responsible for its wound-healing properties.28
Studies have shown that individual comfrey plants within a population can vary
wildly in biochemical composition, particularly in respect to their
pyrrolizidines,103 but alkaloid content also varies within different
tissues of the same plant.105 Numerous sources thus caution that
comfrey should be used externally only,28, 106 but that it should
not be used for an extended period on broken or abraded skin, due to the
presumed increase in absorption of the pyrrolizidine alkaloids. For external
use of comfrey herb and leaf, the German Commission E notes the following cautions:
application should only occur on intact skin; during pregnancy use only after
consultation with a physician; daily applied dosage should not exceed 100 µg
(mcg) of pyrrolizidine alkaloids with 1,2-unsaturated necine structure,
including their N-oxides; and duration of use should not exceed 4-6 weeks per
year.70 Due to all the concerns associated with this remedy, and
considering that multiple safer herbal alternatives are available, this plant
is not recommended for the treatment of TD.102
Flax
Native Americans had several uses for flax (Linum spp. L., Linaceae), and they found it to be
especially effective in treating swellings of wide variety.7, 8 Flax
has been utilized for thousands of years as an oil and then fiber plant by
peoples of Eurasia,51 but only in the last two thousand years as a
medicinal plant.28, 52 The healing powers of the plant lie in its
seed, also known as linseed, which were recognized by the Greeks as being
useful in treating inflammation; today, herbalists use the plant for the same
indications.22, 26, 28, 70, 94, 107
The majority of medical data on flax pertain to its use in
cancerous, cardiovascular, or digestive conditions.24, 26 One study
showed that flaxseed oil taken orally inhibited synthesis of IL-1beta and
TNF-alpha in human volunteers.108 As these are pro-inflammatory
cytokines, long-term ingestion of flaxseed oil may alleviate inflammatory
conditions. For dermatitis, flax is applied externally as a cataplasm.24,
70 These observations suggest that flax may alleviate skin inflammation
and possibly be successful in the treatment of TD. Adverse reactions after oral
ingestion have been reported.109
Goldenseal
Native Americans found goldenseal (Hydrastis canadensis L., Ranunculaceae) root to be useful in treating
inflammation among other conditions.7, 27 For the treatment of TD,
goldenseal is sometimes used in ointments and salves on the wound site itself,
or the dried powder or a tea from the root is employed to wash the affected
area. Usually the roots and rhizomes are used. Berberine and other isoquinoline
alkaloids (such as hydrastine)27 have received much attention for
their antibacterial properties.56, 110, 111 From the limited studies
available, one can speculate that goldenseal’s potential role in mitigating
inflammation may be more indirect in that it protects damaged skin from
becoming infected,24 rather than directly healing the wound. One in
vitro study found that isolated berberine may have deleterious effects on
cultured skin cells in the presence of light,112 but these results
have not been corroborated by in vivo or clinical data.
Mullein
Mullein (Verbascum thapsus L., Scrophulariaceae) is a native of Europe that was only introduced to
North America in the last several centuries.80 However, its
medicinal properties have been known since antiquity and have been utilized by
a variety of cultures for inflammatory disorders.19, 94 Subsequent
to its introduction, Native Americans quickly adopted mullein as an herbal
remedy for bruises and wounds of all types (among other uses).7
Both flowers and leaves are employed medicinally, but
usually the former are preferred.27 In the treatment of TD, juice
from the leaves may be applied raw to the skin, or an extract or ointment may
be made from the juices and applied topically. Decoctions and infusions of the
plant (for example, infusing inflorescences in olive oil)27, 28 have
generally proved to be most efficacious.113 Only in recent years
have these properties been corroborated scientifically; a review and further
data have been published.113 While results of recent studies are
positive, more work needs to be conducted on mullein’s anti-inflammatory
activities; a lack of data precludes any statement of its efficacy in the
treatment of TD. No adverse effects from topical usage have been reported.
Myrrh
The common name “myrrh” (Commiphora spp.
Jacq., Burseraceae) can be applied to several species of the genus Commiphora, most commonly C. molmol, C. myrrha , C. guidotti, and C.
mukul. Sometimes members of the genus Balsamodendron
are also included. When indicated for TD,
myrrh is typically found as one component in multi-component salves or oils.
Myrrh has been used historically in many cultures to treat skin disorders as
well as other conditions,52,56, 70,114-116 and several studies have
demonstrated myrrh’s anti-inflammatory,57,115-117 analgesic,117
anaesthetic,118 and anti-microbial properties.57,115,118 No
study has yet examined myrrh’s efficacy in healing TD. However, the
available evidence would indicate that myrrh is an ideal candidate for treating
this condition. Allergic reactions from this plant have been reported.119,
120
Conclusions
An extraordinary number
of herbal remedies have been recommended historically and contemporarily, in
both the popular and scientific literature, for treating TD. These remedies
vary in efficacy and safety. At this time, the current information available on
these herbs suggests that aloe, echinacea, witch hazel, and possibly plantain,
mullein, and myrrh are the most promising remedies for TD. All of these plants
have been used as broad-spectrum cures for skin disorders. Surprisingly,
jewelweed, the herb most commonly associated with TD treatment, has no support
in the scientific literature.
A resounding theme that emerges from a consideration of the
scientific data en total is that very little scientific investigation has been
conducted regarding herbal remedies for TD. Several studies in the
mid-twentieth century explored herbal remedies in this capacity but yielded
disappointing results, which may explain why no recent research has been
conducted. However, the recent and continuing renaissance of herbal medicine,
mirrored by research funds made available from interested commercial,
government, and research institutions, provide conditions favorable for new
research in this field.
David S. Senchina is a doctoral candidate at Iowa State
University. His research is multidisciplinary, encompassing botany, immunology,
and exercise science. One of his current projects is to document the
pollination ecology of poison ivy (Toxicodendron radicans) through field studies. He has written both original
research articles and reviews on poison ivy for journals such as Wildflower, Vulpia,
and Coleopterists Bulletin.
References
1. Armstrong
WP, Epstein WL. Poison oak: more than just scratching the surface. HerbalGram.1995;No. 34:36-42.
2. Gillis
WT. The systematics and ecology of poison-ivy and the poison oaks (Toxicodendron, Anacardiaceae). Rhodora. 1971;73(793-796):72-159,161-237,370-443,465-540.
3. Baer
H. Chemistry and immunochemistry of poisonous Anacardiaceae. Alt Med Rev. 1986;4(2):152-159.
4. Evans
WC. Trease and Evans’ Pharmacognosy. 14th ed. London: W. B. Saunders Co. Ltd.; 1996.
5. Bruneton
J. Pharmacognosy: Phytochemistry, Medicinal Plants. 2nd ed. Secaucus, NJ, USA/Hampshire, UK: Lavosier/Intercept
Ltd.; 1999.
6. Senchina
DS. Poison ivy: painful to touch, painful to classify. Wildflower. 2003;19(3):16-18.
7. Moerman
DE. Native American Ethnobotany.
Portland, Oregon: Timber Press; 1998.
8. Kindscher
K. Medicinal Wild Plants of the Prairie: An Ethnobotanical Guide. Lawrence, Kansas: University Press of Kansas; 1992.
9. Sollmann
T. A Text-Book of Pharmacology and Some Allied Sciences. Philadelphia & London: W. B. Saunders & Company;
1901.
10. Association
AP. The Pharmaceutical Recipe Book. 2nd ed.
USA: American Pharmaceutical Association; 1936.
11. Culbreth
DMR. A Manual of Materia Medica and Pharmacology. 5th ed. Philadelphia & New York: Lea & Febiger;
1910.
12. Kavasch
EB, Baar K. American Indian Healing Arts.
New York: Bantam Books; 1999.
13. Gibson
MR, Maher FT. Activity of jewelweed and its enzymes in the treatment of Rhus
dermatitis. J Am Pharm Assoc, Sci Ed.
1950;39(5):294-296.
14. Guin
JD, Reynolds R. Jewelweed treatment of poison ivy dermatitis. Contact
Dermatitis. 1980;6(4):287-288.
15. Long
D, Ballentine NH, Marks JGJ. Treatment of poison ivy/oak allergic contact
dermatitis with an extract of jewelweed. Am J Contact Dermat. 1997;8(3):150-153.
16. Zink
BJ, Otten EJ, Rosenthal M, Singal B. The effect of jewel weed in preventing
poison ivy dermatitis. J Wilderness Med. 1991;2(3):178-182.
17. Lipton
RA. The use of Impatiens biflora
(jewelweed) in the treatment of Rhus dermatitis. Ann Allergy. 1958;16(5):526-527.
18. Oku
H, Ishiguro K. Cyclooxygenase-2 inhibitory 1,4-naphthoquinones from Impatiens
balsamina L. Biol Pharm Bull. 2002;25(5):658-660.
19. Sanders
J. The Secrets of Wildflowers. Guilford,
Connecticut: Lyons Press; 2003.
20. Gibbons
E. Stalking the Healthful Herbs.
Chambersburg, Pennsylvania: Alan C. Hood & Company, Inc.; 1989.
21. Meyer
JE. The Herbalist. 1960.
22. Duke
JA. The Green Pharmacy. Emmaus,
Pennsylvania: Rodale Press; 1997.
23. Robbers
JE, Tyler VE. Tyler’s Herbs of Choice: The Therapeutic Use of
Phytomedicinals. New York: Haworth Press,
Inc.; 1998.
24. Blumenthal
M, Hall T, Goldberg A, Brinckmann J, Wollschlaeger B. The ABC Clinical Guide
to Herbs. Austin, Texas: American Botanical
Council; 2003.
25. Duckett
S. Plantain leaf for poison ivy. N Engl J Med. 1980;303(10):583.
26. Yarnell
E, Abascal K, Hooper CG. Clinical Botanical Medicine. Larchmont, New York: Mary Ann Liebert, Inc.; 2003.
27. Foster
S. 101 Medicinal Herbs. Loveland,
Colorado: Interweave Press, Inc.; 1998.
28. Potterton
D. Culpepper’s Color Herbal. New York:
Sterling Publishing Co., Inc.; 2002.
29. Herold
A, Cremer L, Calugaru A, Tamas V, Ionescu F, Manea S, Szegli G. Hydroalcoholic
plant extracts with anti-inflammatory activity. Roum Arch Microbiol Immunol. 2003;62(1-2):117-129.
30. Atta
AH, El-Sooud KA. The antinociceptive effect of some Egyptian medicinal plant
extracts. J Ethnopharmacol.
2004;95(2-3):235-238.
31. Gibson
G. Observations on the internal use of the Rhus radicans. Philadelphia Med
Phys J. 1804;1(1):33-35.
32. Aulde
J. Clinical observations on Rhus toxicodendron. Ther Gazette.
1889;13:676-682.
33. Aulde
J. The use of Rhus toxicodendron. Med & Surg Rep. 1890;63:360-365.
34. Lloyd
J. Origin and History of All the Pharmacopeial Vegetable Drugs, Chemicals,
and Preparations with Bibliography. Volume I. Vegetable Drugs. Cincinnati: The Caxton Press; 1921.
35. Royal
G. Rhus tox as an antiseptic. Med Cent. (NY) 1895;3(3):52-53.
36. Kligman
AM. Hyposensitization against Rhus dermatitis. Arch Dermatol. 1958;78(1):47-72.
37. Still
CC. Botany and Healing: Medicinal Plants of New Jersey and the Region. New Brunswick, New Jersey: Rutgers University
Press; 1998.
38. Dakin
R. Remarks on a cutaneous affection, produced by certain poisonous vegetables. Am
J Med Sci. 1829;4:98-100.
39. Kligman
AM. Poison ivy (Rhus) dermatitis. Arch Dermatol. 1958;77(2):149-180.
40. Epstein
WL, Byers VS, Baer H. Induction of persistent tolerance to urushiol in humans. J
Allergy Clin Immunol. 1981;68(1):20-25.
41. Bowser
RT, Baer H. Contact sensitivity and immunologic unresponsiveness in adult
guinea pigs to a component of poison ivy extract, 3-N-pentadecylcatechol. J Immunol. 1963;91:791-794.
42. Epstein
WL, Baer H, Dawson CR, Khurana RG. Poison ivy hyposensitization: evaluation of
purified urushiol. Arch Dermatol.
1974;109(3):356-360.
43. Watson
ES, Murphy JC, Wirth PW, Waller CW, Elsohly MA. Immunologic studies of
poisonous Anacardiaceae: I. Production of tolerance and desensitization to
poison ivy and oak urushiols using esterified urushiol derivatives in guinea
pigs. J Invest Dermatol.
1981;76(3):164-170.
44. Claus
EP. Gathercoal and Wirth Pharmacognosy. 3rd ed. Philadelphia: Lea & Febiger;
1956.
45. Bogue
EE. The durability of the poisonous property of poison ivy, Rhus radicans L. (Rhus toxicodendron L.). Science. 1894;23(581):163.
46. Sasseville
D, Nguyen KH. Allergic contact dermatitis from Rhus toxicodendron in a phytotherapeutic preparation. Contact
Dermatitis. 1995;32(5):182-183.
47. Cardinali
C, Francalanci S, Giomi B, Caproni M, Sertoli A, Fabbri P. Systemic contact
dermatitis from herbal and homeopathic preparations used for herpes virus
treatment. Acta Derm Venereol.
2004;84(3):223-226.
48. Oh
SH, Haw CR, Lee MH. Clinical and immunologic features of systemic contact
dermatitis from ingestion of Rhus (Toxicodendron). Contact Dermatitis. 2003;48(5):251-254.
49. Heald
P, Burton CS, Callaway JL. On eating poison ivy. N C Med J. 1983;44(7):437-438.
50. Tyler
VE. Pharmacognosy. 7th ed. Philadelphia:
Lea & Febiger; 1976.
51. Simpson
BB, Ogorzaly MC. Economic Botany (Plants in Our World). 2nd ed. New York: McGraw-Hill Inc.; 1995.
52. Zohary
M. Plants of the Bible. Cambridge:
Cambridge University Press; 1982.
53. Ross
IA. Medicinal Plants of the World: Chemical Constituents, Traditional and
Modern Medicinal Uses. Volume I. 2nd ed. Totowa, New Jersey: Humana Press; 2003.
54. Vogler BK, Ernst
E. Aloe vera: a systematic review of its clinical effectiveness. Br J Gen
Pract. 1999;49(447):823-828.
55. Capasso
F, Borrelli F, Capasso R, Di Carlo G, Izzo AA, Pinto L, Mascolo N, Castaldo S,
Longo R. Aloe and its therapeutic use. Phytother Res. 1998;12(S1):S124-S127.
56. Foster
S, Tyler VE. Tyler’s Honest Herbal.
Binghamton, New York: Haworth Press, Inc.; 1998.
57. Atta
AH, Alkofahi A. Anti-nociceptive and anti-inflammatory effects of some
Jordanian medicinal plant extracts. J Ethnopharmacol. 1998;60(2):117-124.
58. Vazquez
B, Avila G, Segura D, Escalante B. Anti-inflammatory activity of extracts from Aloe
vera gel. J Ethnopharmacol. 1996;55(1):69-75.
59. Tan
BK, Vanitha J. Immunomodulatory and antimicrobial effects of some traditional
Chinese medicinal herbs: a review. Curr Med Chem. 2004;11(11):1423-1430.
60. Ernst
E. Adverse effects of herbal drugs in dermatology. Br J Dermatol. 2000;143(5):923-929.
61. Morrow
DM, Rapaport MJ, Strick R. Hypersensitivity to aloe. Arch Dermatol. 1980;116(9):1064-1065.
62. Flannery
M. From Rudbeckia to Echinacea: the emergence of the purple coneflower in modern
therapeutics. HerbalGram.
2000;No. 51:28-33.
63. Borchers
AT, Keen CL, Stern JS, Gershwin ME. Inflammation and Native American medicine:
the role of botanicals. Am J Clin Nutr.
2000;72(2):339-347.
64. Kindscher
K. Ethnobotany of purple coneflower (Echinacea angustifolia, Asteraceae) and other Echinacea species. Econ Bot. 1989;43(4):498-507.
65. Barrett
B. Echinacea: a safety review. HerbalGram.
2003;No. 57:36-39.
66. Barrett
B. Medicinal properties of Echinacea: a
critical review. Phytomedicine.
2003;10(1):66-86.
67. Hobbs
C. Echinacea: The Immune Herb. Santa
Cruz, California: Botanica Press; 1990.
68. Hobbs
C. Echinacea: a literature review. HerbalGram. 1994;No. 30:33-48.
69. Ross
IA. Medicinal Plants of the World: Chemical Constituents, Traditional and
Modern Medicinal Uses. Volume II. Totowa,
New Jersey: Humana Press; 2003.
70. Blumenthal
M, Busse WR, Goldberg A, Gruenwald J, Hall T, Riggins CW, Rister RS (eds.). S.
Klein and R. S. Rister (trans.). The Complete German Commission E
Monographs—Therapeutic Guide to Herbal Medicines. Austin, Texas: American Botanical Council; 1998.
71. Senchina
DS, McCann DA, Asp JM, Johnson JA, Cunnick JE, Kaiser MS, Kohut ML. Changes in
immunomodulatory properties of Echinacea spp. root infusions and tinctures
stored at 4°C for four days. Clin
Chim Acta. In press.
72. Tragni
E, Galli CL, Tubaro A, Del Negro P, Della Loggia R. Anti-inflammatory activity
of Echinacea angustifolia fractions
separated on the basis of molecular weight. Pharmacol Res Comm 1988;20(Supplement 5):87-90.
73. Tubaro
A, Tragni E, Del Negro P, Galli CL, Della Loggia R. Anti-inflammatory activity
of polysaccharidic fraction of Echinacea angustifolia. J Pharm Pharmacol. 1987;39(7):567-569.
74. Tragni
E, Tubaro A, Melis S, Galli CL. Evidence from two classic irritation tests for
an anti-inflammatory action of a natural extract, Echinacina B. Food Chem
Toxicol. 1985;23(2):317-319.
75. Speroni
E, Govoni P, Guizzardi S, Renzulli C, Guerra MC. Anti-inflammatory and
cicatrizing activity of Echinacea pallida
Nutt. root extract. J Ethnopharmacol. 2002;79(2):265-272.
76. Raso
GM, M P, Di Carlo G, Esposito E, Pinto L, Meli R. In-vivo and in-vitro
anti-inflammatory effect of Echinacea purpurea and Hypericum perforatum.
J Pharm Pharmacol.
2002;54(10):1379-1383.
77. Muller-Jakic
B, Breu W, Probstle A, Redl K, Greger H, Bauer R. In vitro inhibition of cyclooxygenase and 5-lipoxygenase by
alkamides from Echinacea and Achillea species. Planta Med. 1994;60(1):37-40.
78. Facino
RM, Carini M, Aldini G, Saibene L, Pietta P, Mauri P. Echinacoside and caffeoyl
conjugates protect collagen from free radical-induced degradation: a potential
use of Echinacea extracts in the
prevention of skin photodamage. Planta Med. 1995;61(6):510-514.
79. Briskin
DP. Medicinal plants and phytomedicines. Linking plant biochemistry and
physiology to human health. Plant Physiol.
2000;124(2):507-514.
80. Gleason
HA, Cronquist A. Manual of Vascular Plants of Northeastern United States and
Adjacent Canada. Bronx, New York: New York
Botanical Garden; 1991.
81. Iscan
G, Kirimer N, Kurkcuoglu M, Baser KHC, Demirci F. Antimicrobial screening of Mentha
piperita essential oils. J
Agric Food Chem.
2002;50(14):3943-3946.
82. Spirling
LI, Daniels IR. Botanical perspectives on health peppermint. J R Soc Health. 2001;121(1):62-63.
83. Alakbarov
F. Aromatic herbal baths of the ancients. HerbalGram. 2003;No. 57:40-49.
84. Mimica-Dukic
N, Bozin B, Sokovic M, Mihajlovic B, Matavulj M. Antimicrobial and antioxidant
activities of three Mentha species
essential oils. Planta Med.
2003;69(5):413-419.
85. Korting
HC, Schafer-Korting M, Hart H, Laux P, Schmid M. Anti-inflammatory activity of Hamamelis distillate applied topically to the skin. Eur
J Clin Pharm. 1993;44(4):315-318.
86. Hughes-Formella
BJ, Filbry A, Gassmueller J, Rippke F. Anti-inflammatory efficacy of topical
preparations with 10% Hamamelis
distillate in a UV erythema test. Skin Pharmacol Appl Skin Physiol. 2002;15(2):125-132.
87. Korting
HC, Schafer-Korting M, Klovekorn W, Klovekorn G, Martin C, Laux P. Comparative
efficacy of Hamamelis distillate and
hydrocortisone cream in atopic eczema. Eur J Clin Pharm. 1995;48(5):461-465.
88. Habtemariam
S. Hamamelitannin from Hamamelis virginiana
inhibits the tumor necrosis factor-[alpha] (TNF)-induced endothelial cell death
in vitro. Toxicon.
2002;40(1):83-88.
89. Pereira
da Silva A, Rocha R, Silva CML, Mira L, Duarte MF, Florencio MH. Antioxidants
in medicinal plant extracts. A research study of the antioxidant capacity of Crataegus, Hamamelis, and Hydrastis. Phytother
Res. 2000;14(8):612-616.
90. Choi
HR, Choi JS, Han YN, Bae SJ, Chung HY. Peroxynitrite scavenging activity of
herb extracts. Phytother Res.
2002;16(4):364-367.
91. Granlund
H. Contact allergy to witch hazel. Contact Dermatitis. 1994;31(3):195.
92. Khanna
N, Datta Gupta S. Rejuvenating facial massage—bane or boon? Int J Dermatol. 2002;41(7):407-410.
93. Holetz
FB, Pessini GL, Sanches NR, Cortez DA, Nakamura CV, Filho BP. Screening of some
plants used in the Brazilian folk medicine for the treatment of infectious
diseases. Mem Inst Oswaldo Cruz.
2002;97(7):1027-1031.
94. Spoerke
DGJ. Herbal Medications. Santa Barbara,
California: Woodbridge Press; 1980.
95. Lin
SC, Lin CH, Lin CC, Lin YH, Chen CF, Chen IC, Wang LY. Hepatoprotective effects
of Arctium lappa Linne on liver injuries
induced by chronic ethanol consumption and potentiated by carbon tetrachloride.
J Biomed Sci. 2002;9(5):401-409.
96. Cho
MK, Jang YP, Kim YC, Kim SG. Arctigenin, a phenylpropanoid
dibenzylbutyrolactone lignan, inhibits MAP kinases and AP-1 activation via
potent MKK inhibition: The role in TNF-alpha inhibition. Int
Immunopharmacol. 2004;4(10-11):1419-1429.
97. Sasaki
Y, Kimura Y, Tsunoda T, Tagami H. Anaphylaxis due to burdock. Int J
Dermatol. 2003;42(6):472.
98. Rodriguez
P, Blanco J, Juste S, Garces M, Perez R, Alonso L, Marcos M. Allergic contact
dermatitis due to burdock (Arctium lappa).
Contact Dermatitis.
1995;33(2):134-135.
99. Rhoads
PM, Tong TG, Banner WJ, Anderson R. Anticholinergic poisonings associated with
commercial burdock root tea. J Toxicol Clin Toxicol. 1984-5;22(6):581-584.
100. Bryson PD, Watanabe AS, Rumack BH, Murphy RC. Burdock
root tea poisoning. Case report involving a commercial preparation. JAMA. 1978;239(20):2157.
101. Fletcher CF, Cantwell JD. Burdock root poisoning. JAMA. 1978;240(15):1586.
102. Mahady GB, Fong HHS, Farnsworth NR. Botanical
Dietary Supplements: Quality, Safety, and Efficacy. Lisse, The Netherlands:
Swets & Zeitlinger; 2001.
103. Stickel F, Seitz HK. The efficacy and safety of
comfrey. Public Health Nutr.
2000;3(4A):501-508.
104. Ahmad VU, Noorwala M, Mohammed FV, Sener B. A new
triterpene glycoside from the roots of Symphytum officinale. J Nat Prod. 1993;56(3):329-334.
105. Awang D. Comfrey update. HerbalGram. 1991;No. 25:20-23.
106. Oberlies NH, Kim NC, Brine DR, Collins BJ, Handy RW,
Sparacino CM, Wani MC, Wall ME. Analysis of herbal teas made from the leaves of
comfrey (Symphytum officinale):
Reduction of N-oxides results in order of magnitude increases in the measurable
concentration of pyrrolizidine alkaloids. Public Health Nutr. 2004;7(7):919-924.
107. Haggerty W. Flax: ancient herb and modern medicine. HerbalGram. 1999;No. 45:51-57.
108. Caughey GE, Mantzioris E, Gibson RA, Cleland LG, James
MJ. The effect on human tumor necrosis factor [alpha] and interleukin 1[beta]
production of diets enriched in n-3 fatty acids from vegetable oil or fish oil.
Am J Clin Nutr. 1996;63(1):116-122.
109. Leon F, Rodriguez M,
Cuevas M. Anaphylaxis to Linum. Allergol Immunopathol (Madr.). 2003;31(1):47-49.
110. Anonymous.
Berberine. Alt Med Rev.
2000;5(2):175-177.
111. Scazzocchio F, Cometa MF, Tomasinni L, Palmery M.
Antibacterial activity of Hydrastis canadensis extract and its major isolated alkaloids. Planta Med. 2001;67(6):561-564.
112. Inbaraj J, Kukielczak B, Bilski P, Sandvik S, Chignell
C. Photochemistry and photocytotoxicity of alkaloids from goldenseal (Hydrastis
canadensis L.) 1. Berberine. Chem
Res Toxicol. 2001;14(11):1529-1534.
113. Turker AU, Camper ND. Biological activity of common
mullein, a medicinal plant. J Ethnopharmacol. 2002;82(2-3):117-125.
114. Thulin M, Claeson P. The botanical origin of scented
myrrh (bissabol or habak hadi). Econ Bot.
1991;45(4):487-494.
115. El Ashry ESH, Rashed N, Salama OM, Saleh A. Components,
therapeutic value and uses of myrrh. Pharmazie. 2003;58(3):163-168.
116. Tipton DA, Lyle B, Babich H, Dabbous MK. In vitro
cytotoxic and anti-inflammatory effects of myrrh oil on human gingivial
fibroblasts and epithelial cells. Toxicol In Vitro. 2003;17(3):301-310.
117. Dolara P, Luceri C, Ghelardini C, Monserrat C, Aiolli
S, Luceri F, Lodovici M, Menichetti S, Romanelli MN. Analgesic effects of
myrrh. Nature. 1996;379(6560):29.
118. Dolara P, Corte B, Ghelardini C, Pugliese AM, Cerbai E,
Menichetti S, Lo Nostro A. Local anesthetic, antibacterial, and antifungal
properties of sesquiterpenes from myrrh. Planta Med. 2000;66(4):356-358.
119. Gallo R, Rivara G, Cattarini G, Cozanni E, Guarrera M.
Allergic contact dermatitis from myrrh. Contact Dermatitis. 1999;41(4):230-231.
120. Al-Suwaiden SN, Gad el Rab MO, Al-Fakhiry S, Al Hoqail
IA, Al-Mazaid A, Sherif AB. Allergic contact dermatitis from myrrh, a topical
herbal medicine used to promote healing. Contact Dermatitis. 1999;39(3):137.
Identification of the Most Common Toxicodendrons in North
America
Eastern poison ivy (Toxicodendron radicans): Climbing vine or shrub
with highly variable leaf morphology (structure and form), though leaves typically
have 3 leaflets. Distinguished from benign trifoliates by its aerial roots and
alternate leaves. Nine subspecies. Eastern and southern U.S.
Atlantic poison oak (Toxicodendron pubescens): Nonclimbing shrub. The epithet pubescens refers to
its hairy fruits and leaves, features that best distinguish it from other Toxicodendron species.
Typically a denizen of nutrient-poor, dry soil habitats. Often confused with
white oaks. Eastern and southern U.S.
Western poison ivy (Toxicodendron rydbergii): Nonclimbing shrub. Distinguished from other Toxicodendron species
in bearing spoon-shaped leaflets (leaflets bent upwards at the midrib); never
producing aerial roots; and having hairless, long petioles (stalks that attach
leaves to the stem). Central and northern U.S. into southern Canada.
Pacific poison oak (Toxicodendron
diversilobum): Climbing or
nonclimbing vine or shrub. Most easily distinguished by its geography: far
western U.S. (California, Oregon, and Washington). As its name suggests, this
species is highly diverse in its leaf morphology (including lobes), often
resembling west coast oak species. Usually trifoliate (having 3 leaves).
Poison sumac (Toxicodendron vernix): Nonclimbing tall shrub or tree. Pinnately
(branching from each side of a common axis) compound leaves with 7-13 leaflets.
Differs from benign sumacs in midvein color (typically bright red), smooth leaf
margin, and preference for wet soil habitats. Eastern U.S. Photo Robert H.
Mohlenbrock @ USDA-NRCS PLANTS Database.
|