Issue: 72 Page: 32-46
Integrating Recent Knowledge about the Genus Echinacea: Morphology, Molecular Systematics, Phytochemistry
HerbalGram. 2006; 72:32-46 American Botanical Council
Integrating Recent Knowledge about the Genus Echinacea: Morphology,
Molecular Systematics, Phytochemistry
by Bernard R. Baum, PhD; Shannon E. Binns, PhD; and John T. Arnason, PhD
Abstract
This article summarizes the authors’ recent research on Echinacea
published in various refereed journals with an emphasis on a new taxonomy.
The taxonomy that most people are familiar with is that of McGregor, established
in 1968. In these new studies, the authors recognize 4 species in Echinacea
and have fitted most of McGregor’s species as varieties under E.
pallida and E. atrorubens, whereas E. purpurea and E.
laevigata remain as before without varieties. The authors’ studies
on genomics and phytochemistry have lent support to this taxonomic scheme.
This article contains an identification key to the 4 species and to the varieties
within E. atrorubens and E. pallida.
Background
Up until the 1960s, the taxonomy of the genus Echinacea was based
on specimens that were collected from parts, but not all, of its natural geographical
range. Further, before the chemistry of the 1980s and the molecular
biology of the 1990s, Echinacea’s taxonomic groupings were based
on morphology first and subsequently on cytological analyses. For instance,
Cronquist described 4 species (and one variety) from his morphological observations
of herbarium specimens (including the actual type specimens associated
with scientific names).1
In 1968, R.L. McGregor embarked on a 15-year odyssey studying wild Echinacea
plants from populations throughout the entire geographical range. His biosystematic
studies included the investigation of macro- and micro-morphological traits
under a common garden design, and he included some cytological comparisons
and some anatomical traits, while making inferences about phylogenetic history,
relating to evolutionary development in the genus. McGregor recognized 9 species
and 4 varieties.2 He proposed that there may be extensive genetic
variation within certain wild populations of a single species or variety and
that further genetic studies were indicated.
Evidence of Phenotypic Variation
Many have relied on McGregor’s identification keys to the wild species
and varieties.2 For example, during the herbal medicine boom of
the early 90s, botanists, conservationists, and diggers (wildcrafters) used
them. Many reportedly found that there was such a large amount of variation
between plants of a single population in the genus Echinacea, and even
between plants of the same age cultivated in a greenhouse, that they were
unable to identify the plants confidently. Furthermore, the market
demand at that time increased the value of wild roots from Echinacea angustifolia
(and later from E. pallida roots) as well as the aerial parts of
E. purpurea. There was a dire need for rigorous and accurate morphological
identification so dealers could provide certified authentic Echinacea
to their customers. One solution to the taxonomic problem was the work
of Bauer and Wagner; they provided chemical profiles of some secondary metabolites,
which were used to distinguish between Echinacea and non-Echinacea
(Parthenium integrifolium) dried samples, and they offered some
possible means to distinguish between the different species and varieties
as well.3 Bauer and Wagner determined that E. pallida (Nutt.)
Nutt. var. pallida, Asteraceae [syn. = E. pallida (Nutt.) Nutt.]
was being cultivated and sold erroneously as E. pallida (Nutt.) Nutt.
var. angustifolia (DC.) Cronq. [syn. = E. angustifolia DC.
var. angustifolia]. Building on Bauer and Wagner’s discovery
of potential chemotaxonomic traits in the commercial species, we undertook
a large-scale taxonomic molecular and phytochemical revision. Our goal was
to ensure more accurate botanical identification of all the different Echinacea
taxa for reasons of safety in the supply chain for phytomedicines and for
reasons of wild rare species conservation.
Conservation of the natural Echinacea resources across North America
has socio-political implications due to issues of private and public land
tenure, especially on Aboriginal (i.e., Native American) land reserves. Governance
of lands and natural resources tends to vary at the federal, state/provincial,
or regional levels in both Canada and the United States. The majority (>90%)
of natural Echinacea populations occur in the United States (see Figure
1 below), where there is a National Germplasm Conservation Program that addresses
all Echinacea taxa among other resources and threatened species in
collaboration with the Nature Conservancy, the State Departments of Natural
Heritage/Conservation, and the US Department of Agriculture (USDA).
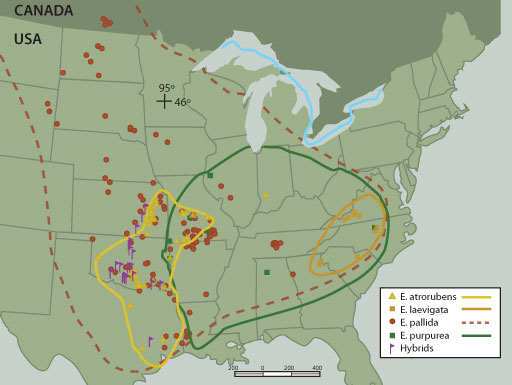 |
| Figure 1. Map of Echinacea species sampled throughout most
of the range of the native populations of this genus in 1998-1999 for
several integrated studies presented herein (reprinted from Binns et
al4). |
Morphological Systematics
In an attempt to rectify the situation of poor botanical identification
methods with Echinacea on the market, we studied 110 wild populations
(see Figure 1 below) to gauge the extent of variation between plants in a
population and between populations in a species. Our objective was to investigate
taxonomic groupings based on the degree of morphological similarity between
plants, and to test the statistical significance of our resulting groupings
using morphometric tools.
Methods
Natural populations were taxonomically identified in the field according
to McGregor,2 and transplanted to a greenhouse for morphometric
data collection.4,5,6 We measured 74 traits for over 300 specimens,
which allowed us to calculate the statistical index of similarity between
(1) individual plants (assuming no prior taxonomic groups), and (2) McGregor’s
taxonomic groups. We used a Gower coefficient of similarity (a biostatistical
measuring tool),7 followed by several clustering methods, and canonical
discriminant analyses to assess the groupings.4
Findings
The morphometric analyses supported 2 acceptable cluster solutions. The
first strongly supported 2 major taxa within Echinacea, which we determined
to be at subgenus level. The species known currently as E. purpurea
(L.) Moench was the sole taxon in Echinacea subgenus Echinacea
which contains only E. purpurea, whereas all other infrageneric taxa
were in Echinacea subgenus Pallida. The second most acceptable
cluster solution supported 4 taxa, which we determined to be at the species
level: (1) E. purpurea [= Echinacea purpurea (L.) Moench nom.
cons. prop.],8 (2) E. laevigata [= E. laevigata (Boynton
& Beadle) Blake], (3) E. atrorubens, and (4) E. pallida.
Therefore, we effectively re-classified the genus Echinacea into 2
subgenera, one with a single species in it, and the other having 3 species.
Our results also supported an 8 cluster solution using McGregor’s identification
keys.2 The 8 groups correspond to varieties within 2 species (see
Table 1). The revised taxonomy recognizes all of McGregor’s taxa, except
for one variety, E. angustifolia DC. var. strigosa McGregor,
which was not distinct from E. pallida var. angustifolia. This
putative variety may be a morphotype that resulted from introgression [movement
of alleles from one taxon to another through hybrid intermediates, usually
found in populations bordering and/or overlapping each other], and it shows
the same phenotype [the visible, measurable characteristics, which may vary
independent of genetic makeup] in similar ecological zones.8
Identification Key for Echinacea Species and Varieties
A dichotomous identification key is used by biologists to identify organisms
to different levels, such as family, genus, species, or variety. It is designed
to list traits of organisms as a series of paired choices that lead progressively
to identification of the organism. Not all keys lead to the same level of
taxonomic identification, and it is important to have all traits match the
key statement that is chosen for any given specimen in order to arrive at
the most accurate identification of that organism. In the key below, one may
proceed to take a plant or specimen in question and choose between the pair
of statements numbered with “1,” which then leads either to
a choice between a pair of “2” statements (and eventually identification
of plants in the subgenus Pallida), or to identify the plant in question
as subg. Echinacea, E. purpurea (L.) Moench. If one continues
to follow the number at the end of the statement that is true, one will eventually
arrive at the best identification of that particular specimen. Note that if
some traits in the key are not observable in the specimen in question, then
another specimen with those missing organs must be used for the key to function
properly.
1. Basal leaf up to 5 cm wide; cauline leaf 0.5 to 4.5 cm wide; taproot
(may be branching or fusiform); leaf blade trichomes multicellular with knobby
joints; major veins almost parallel from a common origin at the base; 1-3
series of involucral bracts 2. subg. Pallida
1. Basal leaf greater than 5 cm wide; cauline leaf 4.5 to 9 cm wide; fibrous
roots (from a caudex); leaf blade trichomes bicellular with ledge-like joints;
major veins branched; four series of involucral bracts subg. Echinacea,
E. purpurea (L.)
Moench
2. Basal leaf greater than 3 cm wide; basal leaf margin serrate, or dentate;
adaxial leaf blade stalked trichomes absent; stem stalked trichomes absent;
cauline leaf margin serrate
E. laevigata (C.L. Boynton & Beadle) Blake
2. Basal leaf up to 3 cm wide; basal leaf margin entire; adaxial leaf blade
stalked trichomes present; stem stalked trichomes present; cauline leaf margin
entire 3
3. Stem stalked trichomes appressed (strigose); leaf blade stalked trichomes
sparse; leaf marginal trichomes different than blade trichomes (more appressed).
4
4. Ray floret yellow E. atrorubens Nutt.
var. paradoxa (J. B. Norton) Cronq.
4. Ray floret pale pink to purple, or white 5
5. Disk corolla petal fusion more than 3/4 total corolla length; involucral
bract up to 0.2 cm wide; stem branched….E. atrorubens Nutt. var.
atrorubens Cronq.
5. Disk corolla petal fusion less than 3/4 total corolla length; involucral
bract greater than 0.2 cm wide; stem unbranched E. atrorubens Nutt.
var. neglecta (McGregor) Binns B. R. Baum & Arnason
3. Stem stalked trichomes hirsute, or straight pubescent; leaf blade stalked
trichomes dense; leaf marginal trichomes identical to leaf trichomes in type
and habit
6
6. Ray floret up to 4 cm long 7
7. Capitulum up to 2.5 cm wide; involucral bract up to 0.2 cm wide E.
pallida (Nutt.) var. tennesseensis (Beadle) Binns B. R. Baum &
Arnason
7. Capitulum greater than 2.5 cm wide; involucral bract greater than 0.2
cm wide E. pallida (Nutt.) var. angustifolia (DC.) Cronq.
6. Ray floret greater than 4.0 cm 8
8. Fresh pollen white E. pallida (Nutt.) Nutt. var. pallida
8. Fresh pollen yellow, or lemon 9
9. Ray achene trichomes present; stem unbranched. E. pallida (Nutt.)
var. simulata (McGregor) Binns B. R. Baum & Arnason
9. Ray achene trichomes absent; stem branched. E. pallida (Nutt.)
var. sanguinea Gandhi & Thomas
Related information for identification of species and varieties (including
an interactive key, and alternative key with McGregor’s taxonomy) may
be found on the Web site of Agriculture Canada (http://res2.agr.gc.ca/ecorc/echinacea/key-cle_e.htm).
This will eventually be modified online to allow for identification from separate
plant parts. To identify a whole plant, one can choose one of the alternative
descriptions at number 1 and then follow the leads.
Evolutionary hypotheses
The greatest amount of morphological and genetic diversity observed among
geographically-close populations was found in a narrow region of the Great
Plains, which is considered by field botanists to be the center of Echinacea
diversity.4,9 The “center of diversity” spans several
eco-regions that share characteristics of having overlapping biogeoclimatic
“edges,” such as tallgrass prairie abutting limestone upland formations
and/or shortgrass prairies. The following areas are included in the hypothetical
region: Ozark Mountains of Missouri and Arkansas, prairies of Kansas and Oklahoma,
and especially Black Hills of southeastern Oklahoma where suspected hybridization
and introgression may be directing the most active speciation within the genus.4,9
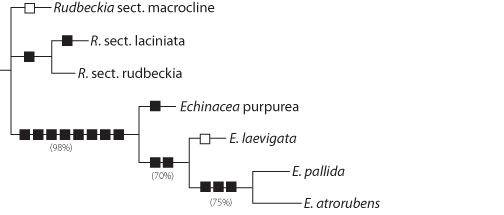 |
| Figure 2. Cladogram of Echinacea species (reprinted from Binns
et al5). A 40-step most parsimonious cladogram representing the monophyletic
genus Echinacea Moench compared to 3 sections of Rudbeckia
in an outgroup. Confidence intervals are indicated in brackets below the
branches (bootstrap values using the 50% majority-rule consensus method).
Cladistic analysis was performed with 36 characters (Binns et al4).
Dark boxes signify synapomorphies and empty boxes signify parallelisms.
|
In our work, the evolutionary relationships between the 4 revised species
were estimated using a cladistic analysis [based on shared, derived characters
which are often also diagnostic] of 36 characters (including some phytochemical
ones). See the cladogram [an evolutionary tree] (see Figure 2 right), where
Echinacea is distinguished phylogenetically from the outgroup [a sister
group] Rudbeckia (98% bootstrap value) [a method for assessing the
statistical significance of the relationships between taxonomic groups, i.e.,
positions of branches in an evolutionary tree]. Within the Echinacea clade,
E. atrorubens and E. pallida share 3 unique, derived characteristics,
and E. purpurea was most basally divergent. Although historically E.
laevigata was confused with E. purpurea,10 current morphometric
results show it to be closely related to E. pallida and E. atrorubens.
In summary, we proposed a hierarchy of 2 subgenera, 4 species and 6 varieties
in the genus Echinacea.4 The 2 subgenera are novel, but
our results confirm the 4 species groups that were first suggested using classical
taxonomic methodology.1 All of our described varieties were previously
either species or varieties according to McGregor.2 Table 2 on
page 37 compares the classifications of both McGregor2 and Cronquist1,11,12
to the revised taxonomy.4
| Table 2. Taxonomic treatments of Echinacea
Moench by McGregor,2 Cronquist,1,11,12 and Binns, Baum, and Arnason.4
Synonyms are in square brackets []. Permission to reprint Binns ©
2001 University of Ottawa. |
| McGregor |
Cronquist |
Binns, Baum, and Arnason |
1. E. angustifolia DC. var. angustifolia
E. angustifolia DC. var. strigosa McGregor |
1. E. pallida (Nutt.) Nutt. var. angustifolia
(DC.) Cronquist
[E. angustifolia DC. var. strigosa McGregor] |
1. E. pallida (Nutt.) Nutt. var. angustifolia
(DC.) Cronquist
E. angustifolia DC. var. strigosa
McGregor] |
| 2. E. tennesseensis (Beadle) Small |
[E. tennesseensis (Beadle) Small] |
E. pallida (Nutt.) Nutt. var. tennesseensis |
| 3. E. pallida (Nutt.) Nutt. |
E. pallida (Nutt.) Nutt. var. pallida |
E. pallida (Nutt.) Nutt. var. pallida |
| 4. E. simulata McGregor |
[E. simulata McGregor] |
E. pallida (Nutt.) Nutt. var. simulata (McGregor)
Binns, B. R. Baum & Arnason |
| 5. E. sanguinea Nutt. |
[E. sanguinea Nutt.] (suggested variety) |
E. pallida (Nutt.) Nutt. var. sanguinea (Nutt.) Gandhi
and Thomas |
| 6. E. atrorubens Nutt. |
2. E. atrorubens Nutt. var. atrorubens |
2. E. atrorubens Nutt. var. atrorubens |
7. E. paradoxa (Norton) Britton var. paradoxa
E. paradoxa (Norton) Britton var. neglecta McGregor |
E. atrorubens var. paradoxa (Norton) Cronquist |
E. atrorubens Nutt. var. paradoxa (Norton) Cronquist
E. atrorubens Nutt. var. neglecta (McGregor) Binns,
B. R. Baum & Arnason |
| 8. E. laevigata (Boynton & Beadle) Blake |
3. E. laevigata (Boynton & Beadle) Blake nom. cons.
prop. |
3. E. laevigata (Boynton & Beadle) Blake nom. cons. prop. |
| 9. E. purpurea (L.) Moench |
4. E. purpurea (L.) Moench nom. cons. prop. |
4. E. purpurea (L.) Moench nom. cons. prop. |
Molecular Systematics Based on DNA Methods of Purple Coneflowers:
Genus Echinacea
How does this genus of Purple Coneflowers fit with the other Coneflower
genera?
Echinacea is a genus classified in the Heliantheae tribe within the
family Asteraceae. Together with other genera in this tribe, Echinacea
plants are popularly known to be among the “Coneflowers.” The
relationship of the genus Echinacea to others has been studied using
techniques which aim to determine the degree of relationship and the probable
evolutionary development of these plants over time. For example, an article
published in 1995 by Urbatsch and Jansen reported restriction site analysis
of the chloroplast genome, which placed the genus in the subtribe Ecliptinae.13
This subtribe is distinct from, yet closely related to, Rudbeckiinae, which
contains the genera Dracopsis, Ratibida, and Rudbeckia.14,15
Subsequently, Urbatsch et al used another approach, nuclear rDNA internal
transcribed spacer (ITS) sequences, to study evolutionary relationships among
the Coneflowers and relatives and also to combine the data with their previous
chloroplast DNA restriction site data.16 They concluded that Echinacea
ought to be classified within the tribe Zinniinae, and that it is definitely
not related to genera in the Rudbeckiinae.
In the cladogram of the combined data they used 6 species (sensu
McGregor 1968) of Echinacea with similar results of relationships among
species as in the chloroplast DNA restriction site data, i.e., that E.
purpurea is closely related to E. paradoxa.
Species and varieties of Echinacea
Using Amplified restriction Fragment Length Polymorphism (AFLP®,
see side bar on page 36), Mechanda et al17 undertook a study
in parallel to Binns et al4 to seek independent support
for the morphologically based classification (including relationships) and
to complement it in 2 respects: (1) to estimate the genetic diversity of the
species and varieties, and (2) to provide means of identification of single
plants by DNA fingerprinting.
Four hundred thirty-five individual plants were sampled from 58 natural
populations representing both the area of distribution and the species and
varieties of both Binns et al4 and McGregor2
classifications. The most notable outcome resulting from the AFLP investigation
was that each individual could be uniquely distinguished by a combination
of presence/absence of a set of 124 fingerprints. The main finding of this
study was support for the 4 species classification of Binns et al,4
but not for all the varieties, most of which were previously recognized by
McGregor as species.2 The species are recognized by a combination
of DNA fingerprints, not by a single or a few single and unique AFLP bands.
Thus, to identify an individual plant to species with AFLP one needs to resort
to more elaborate means, as indicated in Mechanda et al with an example
(refer to Table 11 in their article).17 In the example, 10 DNA
bands from a single primer set are apparently sufficient to identify an unknown
plant, or plant fragment, to one of the 4 recognized species.
| Table 1. Taxonomy of McGregor2 compared
to the revised taxonomy of Binns et al4 for species and varieties of genus
Echinacea. |
| McGregor (1968) |
Binns et al (2002) |
| E. purpurea |
E. purpurea |
| E. pallida |
E. pallida var. pallida |
| E. angustifolia |
E. pallida var. angustifolia |
| E. sanguinea |
E. pallida var. sanguinea |
| E. simulata |
E. pallida var. simulata |
| E. tennesseensis |
E. pallida var. tennesseensis |
| E. atrorubens |
E. atrorubens var. atrorubens |
| E. paradoxa |
E. atrorubens var. paradoxa |
| E. paradoxa var. neglecta |
E. atrorubens var. neglecta |
| E. laevigata |
E. laevigata |
As far as relationships among the 4 species are concerned, although no attempt
was made to use any outgroup (a reference outside Echinacea but close
enough to it) in the AFLP study, E. laevigata and E. purpurea
can be construed as forming a sister group based on the unrooted UPGMA dendrogram
(refer to Figure 4 in Mechanda et al 2004,17 which is not
a true phylogenetic tree). This can easily be seen when moving the branches
of the dendrogram without changing the topology. Based on this the genus Echinacea
consists of 2 parallel pairs: E. purpurea-E. laevigata and E.
atrorubens-E. pallida. This finding supports the gross morphological
similarity seen between at least the first two, since they have sometimes
been confused.8,10
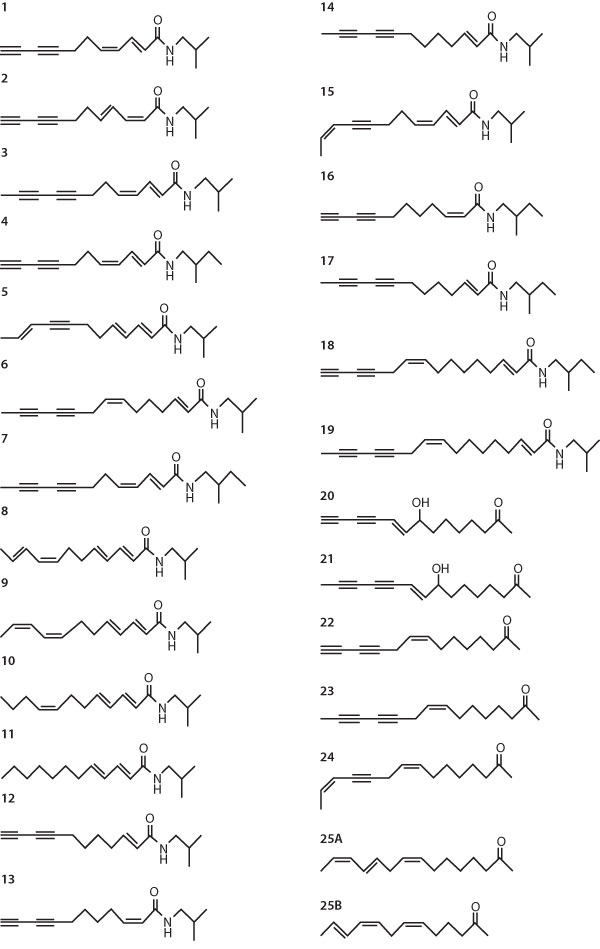 |
| Figure 4. Alkamides and ketoalken/ynes in Echinacea species
and varieties. |
The gene diversity of all the species together in the genus and similarly
for the varieties together (measured on a scale from 0 to 1) was nearly 0.5
for both. The species with highest gene diversity was E. purpurea,
also near 0.5 whereas the 3 other species had lower rates at 0.3. Both E.
purpurea and E. laevigata do not contain varieties. Although the
varieties were not supported by the AFLP results, when analyzed for genetic
diversity, those of E. pallida had greater values than those of E.
atrorubens, with the exception of E. pallida var. tennesseensis
having the lowest genetic diversity (near 0.2), which is understandable due
to its rarity with limited individuals in the populations (E. pallida
var. tennesseensis is currently listed as a federally endangered species
by the US Fish and Wildlife Service18). The genetic variation was
apportioned as follows: 19% among species, 40% among populations within species,
and 41% within populations. In other words, the genetic variation among populations
was found to be about equal to the genetic variation within populations. But
obviously the kind of variation was different since every individual was found
to possess unique fingerprints.
Once you know it’s Echinacea, how do you determine
what kind of Echinacea it is?
There were major difficulties in identification of plant materials that
were reported by wildcrafters, growers, and scientists during the early days
of Echinacea’s boom in the commercial marketplace (see Morphological
Systematics section on page 33). For this reason, 2 collaborating research
teams used modern tools in both morphometric taxonomy4 and molecular
systematics17 to discover which natural taxonomic groups exist
currently in wild plant populations. Mechanda et al distinguished wild
species and varieties in Echinacea using Amplified restriction Fragment
Length Polymorphism (AFLP®).17 This generated results
about relationships between plants and populations based on DNA. Also, it
allowed for independent support for the morphological classification by complementing
it in 2 respects: (1) it estimates the genetic diversity of all types of Echinacea
species and varieties growing in the wild, and (2) it provides a means for
stakeholders to identify and trace single plants by DNA fingerprinting.
How are the different kinds of Echinacea species and varieties
related?
Based on work by Mechanda et al, the genus Echinacea consists
of 2 parallel pairs: E. purpurea-E. laevigata and E. atrorubens-E.
pallida. E. laevigata and E. purpurea can be construed as
forming a sister group based on the unrooted UPGMA dendrogram (see Figure
4 in Mechanda et al 2004, which is not a true phylogenetic tree).17
In this approach, no attempt was made to use any outgroup comparison, and
the tree is not a true phylogenetic tree (as seen by the unchanging topology
when branches in the tree are rotated). This finding supports the gross morphological
similarity seen between E. purpurea and E. laevigata.8,10
Both E. purpurea and E. laevigata do not contain varieties.
The other 2 species, E. pallida and E. atrorubens, each contain
varieties by morphometric classification,4 but classification by
AFLP results did not resolve distinct groups at the variety level. In fact,
genetic diversity was measured on a scale of 0 to 1 and found to be 0.5 among
species, and also 0.5 for all varieties together. The species with highest
gene diversity was E. purpurea, near 0.5, and the 3 other species had
lower rates at 0.3. Varieties of E. pallida had greater diversity ratings
than those of E. atrorubens, with the exception of the rare E. pallida
var. tennesseensis having the lowest genetic diversity (near 0.2).
Although hybrids and hybrid populations were reported by McGregor,2
we were unable to distinguish them from others by genetic diversity measured
with AFLP analysis, although more than one suspected hybrid population in
the field was identified in the morphometric work by Binns et al.4
Other DNA work done on Echinacea
Urbatsch and Jansen only studied 7 of the 9 Echinacea species recognized
by McGregor2 and found that E. purpurea was closely related
evolutionarily to E. atrorubens and E. paradoxa, and that E.
simulata was possibly the more ancestral species.13 Later,
Urbatsch et al reported combined data analysis using 6 species of Echinacea
(sensu McGregor 1968).16 Their cladogram shows similar
relationships among species to those in the Urbatsch and Jansen paper on chloroplast
DNA restriction site data,13 i.e., E. purpurea is closely
related to E. paradoxa.
As part of our AFLP study we found that the AFLP fingerprints were inappropriate
for phylogenetic studies.17 However, Kim et al carried out
a similar AFLP study to ours, with much less sampling to assess phenetic/phylogenetic
relationships among Echinacea species and varieties (sensu McGregor).19
Their results, not surprisingly, did not provide support for the presently
accepted classification by Binns et al.4 One reason for
this is that AFLP data are usually inappropriate for phylogenetic studies
demonstrated on theoretical grounds, as explained by Clark and Lanigan20
regarding RAPD data. Clark and Lanigan’s explanation equally applies
to AFLP data in many respects, including ours (refer to the Discussion section
on “Phylogenetic analysis,” pages 480-481 in Mechanda et al17).
Another reason is that the identification of their material may be questionable,
especially if they relied on McGregor’s keys, which have been problematic
in the past.4 A different investigation was carried out by Kapteyn
et al using DNA-RAPD.21 RAPD has proven to be less amenable
to reproducibility than AFLP. Kapteyn et al used only the 3 main commercial
species (E. angustifolia, E. pallida, and E. purpurea) and their
study remained inconclusive.21
How can DNA markers be used to authenticate sample materials of Echinacea
species and commercial lines (cultivars?) within species?
That authentication of commercial material of Echinacea is of prime
interest to the consumer goes without saying. Authentication is needed to
ensure the correct and proper content of the product. Correct identification
of the plant material constitutes one aspect, and correct phytochemical characterization
of plant extracts is another aspect (i.e., the quantitative analysis of marker
phytochemicals for assurance of safety, quality, and potentially of therapeutic
value). Both are needed in the natural health products industry. The study
by Mechanda et al has shown the potential of correct identification
to species.17 In another study, the use of DNA-based markers to
predict phytochemical profiles in extracts of identical material were assessed
by Baum et al, using AFLP and High Pressure Liquid Chromatography (HPLC).22
In this study, we determined both AFLP DNA fingerprints as well as the quantitative
profiles of 2 marker compounds, namely, cichoric acid (2,3-O-dicaffeoyltartaric
acid) and dodeca-2E, 4E, 8Z, 10E/Z-tetraenoic acid isobutyl amide in over
50 accessions of E. purpurea. This small study has shown the potential
of DNA markers to predict the amount of industry marker chemicals. An extension
of this study or a similar DNA-based study may be needed to distinguish the
true product from adulterants (wrong plant species, wrong plant line, wrong
plant part) and contaminants (like bacterial or fungal, foreign matter).
Phytochemical Variation of Echinacea in Wild Populations
The phytochemistry of Echinacea species is of key importance to the
herbal industry because the phytochemical markers are some of the most easily
identifiable characters for species identification in processed products.
Also, they are biologically active substances that have importance in the
pharmacology of the products. The basic phytochemistry of Echinacea
was undertaken in Germany at a time when there was little interest in North
America in this medicinal endemic prairie genus.3,23 The main groups
of importance are caffeic acid derivatives (CADs) (see Figure 3 below), lipophilic
alkamides (AAs) and ketoalken/ynes (see Figure 4 on page 39), although other
types of secondary metabolites are also found in the genus.
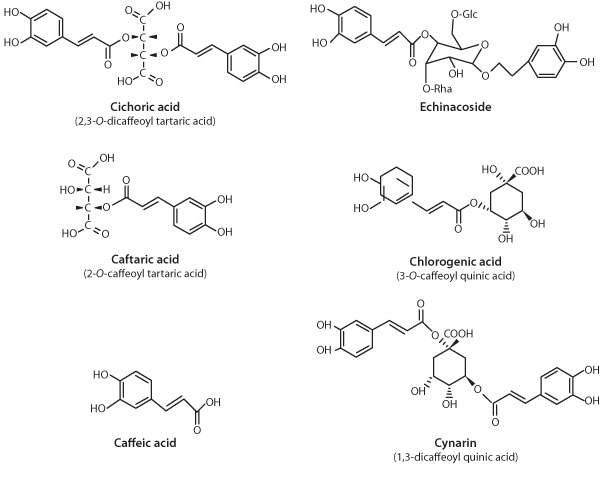 |
| Figure 3. Caffeic acid derivatives in Echinacea species and
varieties. |
It is well known in chemosytematics that individual species are likely to
have their own unique blend of phytochemicals, developed in the co-evolutionarily-driven
progression towards developing novel defense chemicals. Bauer showed that
this principle can be used to distinguish the 3 commercial Echinacea
taxa by HPLC analysis if other species/varieties are not considered as possible
components of a mix.3,23 Moreover, in Echinacea, there is
a high degree of phytochemical redundancy in individual species, i.e., they
contain 5 or more CADs and 10 or more AAs. As a result of phytochemical research,
unique marker phytochemicals have been used qualitatively to certify botanical
identification in the industry. Commercial species/marker relationships used
in industry are as follows: echinacoside as a marker for E. pallida
var angustifolia versus E. purpurea, and the use of ketoalkene/ynes
as markers for E. pallida var pallida.
The recent revision of the genus Echinacea and the detailed study
of phytochemistry of all the species and varieties from wild and cultivated
sources reveal a more complex picture.4 This is important to the
herbal industry for the purpose of assessing contamination of commercial seed
lots with wild species or the use of wildcrafted species of doubtful origin
in the product. The phytochemical variation also supports the morphometric
classification of different taxa within the genus by Binns et al,5
but the identification of all the species and varieties using phytochemistry
is confounded by polyploidy [having more than 2 sets of chromosomes which
are homologous (same genes, not necessarily the same gene products/functions)]
and cannot be achieved solely on the basis of presence or absence of one or
a few compounds. The major findings follow.
The current industry practice, which uses echinacoside as a positive marker
for E. pallida var. angustifolia versus E. purpurea where
it is absent, is an over simplification if all species and varieties are considered.
In fact, echinacoside is present in quantifiable amounts from roots of all
Echinacea taxa except E. purpurea; namely, 3 species and 7 varieties.4,5
Trace amounts of echinacoside were found in E. purpurea, so lack of
this compound is not the best marker. The absence of alkamide 18 was found
to be a more definitive E. purpurea marker.
Ketoalkenes/ynes cannot be taken as definitive markers for E. pallida
var. pallida, as often considered in industry practice. However, they
do appear to be markers for polyploids. They are found not only in E. pallida
var. pallida but also in E. pallida var. simulata
which is sometimes triploid, as well as possibly hybridizing populations of
E. atrorubens var. neglecta and E. atrorubens var. paradoxa.
On a positive note, the species and varieties are readily distinguishable
on the basis of quantitative HPLC (high-performance liquid chromatography)
profiles of the compounds (see Figure 5 on pages 40 and 41 and
Figure 6 on page 42 and 43), but not generally on the presence or absence
of individual compounds; notably, the roots of E. angustifolia and
E. pallida can be differentiated by the occurrence of the CADs, 1,3-0-(cynarin)
and 1,5-0-dicaffeoylquinic acids present only in the former.24
Canonical discriminant analysis revealed that cichoric acid, the diene AAs
1-3 and 7, and ketoalkene 24 were the best taxonomic markers. HPLC profiles
for the lipophilic compounds contain more information because they contain
a larger number of compounds.
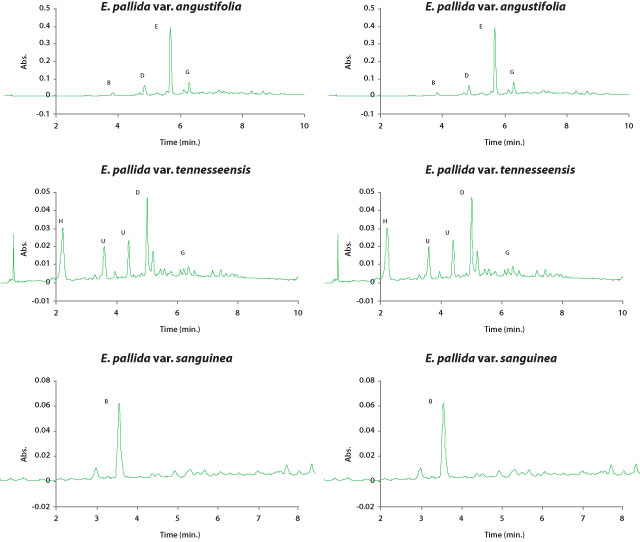
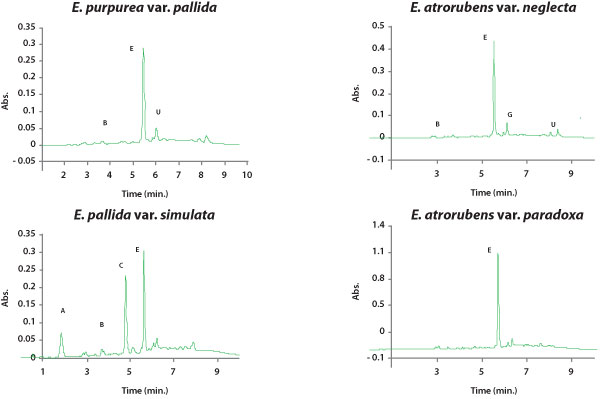
|
| Figure 5. HPLC chromatograms of typical root profiles of hydrophilic
phytochemicals in each Echinacea taxon. Peaks are as follows: (A) caftaric
acid, (B) chlorogenic acid, (C) cichoric acid, (D) cynarin, (E) echinacoside,
(F) cichoric acid methyl ester, (G) rutin, (H) caffeic acid, (U) unconfirmed
(quinoyl), (UC) unconfirmed (di-caffeoyl). For compound structures see
Figure 3. Absorbance was detected at 326 nm. |
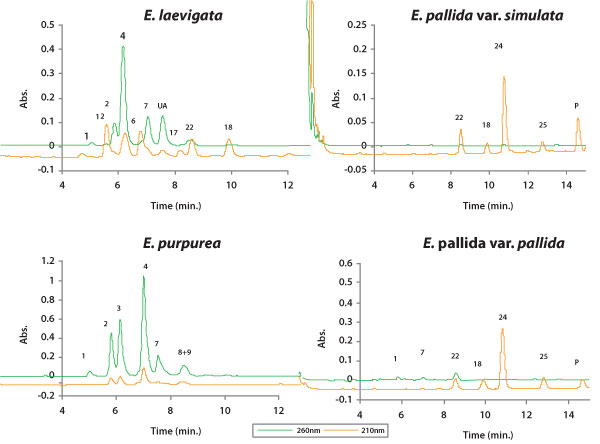
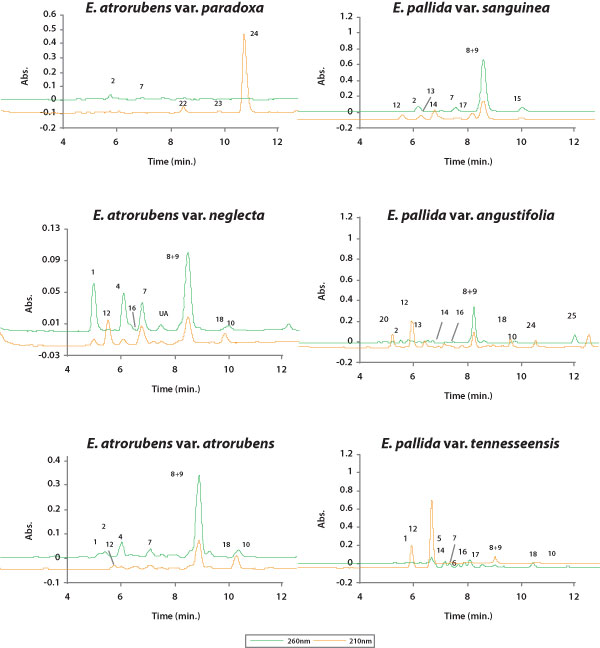
|
| Figure 6. HPLC chromatograms of typical flowerhead profiles of lipophilic
phytochemicals in each Echinacea taxon. Numbered peaks correspond to Figure
4. Lettered peaks are as follows: (P) unreported polyene that resembles
22 by UV-scan, (*P) unreported polyene (UV-scan identical to 24), (UA)
unreported diene alkamide, (UA*) unreported tetraene alkamide. |
Plant age (and plant part) generally changes the expression of compounds.
Young plants expressed lower amounts of alkamides in roots and flowers than
present in older plants. Levels of compounds in young roots can also be increased
significantly by induction with methyl jasmonate, which suggests they are
also inducible by mechanical, insect, or fungal damage.25
In other studies we showed that the quantitative presence of phytochemicals,
such as 8,9 and cichoric acid in mature plants, can be correlated to DNA markers
(AFLPs) across a wide variety of Echinacea germplasm.22
This indicates that DNA markers may be useful in screening germplasm for active
principles where phytochemistry may be less easy to assess.
In another study, Binns et al addressed the question of how much
phytochemical variation is naturally present within and between wild populations.10
This is important for the wild harvest of seed for sale or cultivation, and
the continued wild harvest of Echinacea populations from certain areas
within the native range of these plants. E. pallida var. angustifolia
was chosen for study because it has the largest latitudinal spread of any
species/variety and occurs from Manitoba to Texas. There was significant variation
in AAs and CADs between populations studied, which may support the existence
of distinct chemoraces in this variety.10 Fortunately this variation
is largely quantitative, and does not alter the phytochemical profile needed
to identify the species. Also, since these experiments were conducted on seeds
from the range of this variety, grown in uniform conditions, the variation
measured was more likely to be genotypic rather than phenotypic, or caused
by environmental influences.
Along with (North) American ginseng (Panax quinquefolius L., Araliaceae)
Echinacea is possibly North America’s most important ethnobotanical
product. Since it originates from here, the North American industry must contend
with the genetic diversity associated with the center of origin of this medicinal
crop.
Diversity and rarity in natural populations of Echinacea
species and the case for cultivation of this natural resource
Degradation in wild populations and increased rarity has been observed over
the last few decades during the Echinacea boom. Publicly-acknowledged
rare taxa (and the states in the United States where they naturally grow,
shown parenthetically) include:
E. laevigata (GA, MD, NC, PA, SC, VA) (USFWS, 2004:
first listed 1982, recovery plan enacted in 1995)18
E. pallida var. tennesseensis (TN) (USFWS,
2004 first listed 1979, recovery plan enacted 1989)18
E. pallida var. pallida (MO)
E. pallida var. simulata (MO)
E. pallida var. sanguinea (LA, TX, OK)
E. atrorubens var. paradoxa (MO)
E. atrorubens var. neglecta (TX, OK)
Our taxonomic treatment of the genus did not lead directly to revision or
clarification of protection laws regarding Echinacea. This is largely
because while the taxonomic nomenclature changed, it has not yet been applied
at the practical level of plant identification. Our assessments of diversity
within genus Echinacea (genotypic and phenotypic) should be considered
together with ecological evidence26 in order to guide the taxonomic
and practical protection of species and varieties that are at risk.
Ecophysiology, Competition, and Establishment
E. laevigata and E. pallida var. tennesseensis are
located in marginal and highly vulnerable sites, on calcareous soils, in open
woods, and in cedar barrens. It is likely that these and other “rare”
taxa in this genus arose in Savannahs, which were caused and maintained by
fires set by Native Americans.
In Tennessee, where E. pallida var. tennesseensis is the showy
state wildflower, the ecological status and recovery of this Echinacea
taxon has long been researched.27,28,29,30,31 Recovery
operations in place by US Fish and Wildlife, partnered with the State Department
of Environment and Conservation, include the following measures: restriction
of vehicular traffic, added fencing, limited livestock use of the areas (there
is evidence that cattle graze on plant competitors and thus might increase
seedling establishment and survivorship), public education projects, ecological
monitoring, and acquisition of land by the Nature Conservancy, as well as
by federal and state departments. Very rare populations will be deemed “recovered”
when at least 5 populations are self-sustaining (i.e., stable or increasing
over at least 10 years, with at least 2 juvenile plants for every adult plant).
Through this rigorous recovery activity and monitoring, E. pallida
var. tennesseensis is expected to be down-listed from “endangered”
to “threatened” in January, 2007 (T. Merritt, Personal Communication,
USFWS Cooksville, Tennessee, August 22, 2006).
Is rarity an ecophysiological occurrence?
It was found that E. pallida var. tennesseensis plants are
not highly competitive compared with other glade species, especially under
the effects of allelopathy [release of chemical substances by one species
that inhibit the germination and/or growth of other species of plants] by
species such as: Juniperus virginiana L., Cupressaceae, and Dalea
gattingeri (Heller) Barneby, Fabaceae [syn. Petalostemon gattingeri
Heller].30,32 Moreover, plants of this variety do not have significantly
different ecophysiological requirements in terms of their light and moisture
use.29
Root physiology directly affects competitive abilities. Echinacea
plants are taprooted forbs, which have difficulty establishing in both mixed
and tallgrass prairie due to the competitive advantage of native grasses.
However, E. pallida var. angustifolia, Psoralidium tenuiflorum
(Pursch.) Rydb. Fabaceae (Slimflower scurfpea), Dalea spp. L. Fabaceae
(Prairie clover), and other taprooted forbs generally outperform rhizomatous
forbs, which compete directly with grasses for nutrients.33 Edaphic
constraints [factors pertaining to soil ecological relationships] due to competition
for nutrients and water are higher for rhizomatous forbs.26
There is also evidence that ecological variation (such as edaphic characteristics)
affects genomic variability and/or expression of secondary chemistry. Chemotypes
or chemical races were distinguished for populations of E. pallida
var. angustifolia grown from wild seed,5 despite widespread
and mostly continuous distribution of E. pallida var. angustifolia
in a range of habitats. On the other hand, a significantly lower genetic
diversity was measured in E. pallida var. tennesseensis17,27
and was attributed to possible historical extinction and colonization
events.27 These 2 taxa are distinct but closely related, since
the genetic makeup of E. pallida var. tennesseensis is identical
to that of E. pallida var. angustifolia at 50% of genes studied
by isozyme analysis, and there appears to be a subset of var. angustifolia
alleles at another 28% of E. pallida var. tennesseensis genes
studied.4,27
Restoration of native prairie Echinacea populations is likely to
depend largely on the other plants in the community. Seed recruitment is low.
In some prairie remnants, there is evidence that wild-harvested Echinacea
pallida var. angustifolia can re-sprout from holes where root fragments
are left after digging (K. Kindscher, personal communication, June 5, 1999);
however, the degree to which it may compete for establishment is still under
longer-term study.
E. purpurea has been used for comparisons of competitive abilities
(plant size and reproductive capacity) between wild and cultivated populations.
Snyder et al showed that wild plants tend to have increased vegetative
growth, while cultivated plants display increased reproductive capacity.34
Is rarity a result of human activity?
Wilcove et al implicated habitat degradation in the decline of 85%
of 1880 species of imperiled plants and animals in the United States.35
Thirty-five percent of these were directly linked to commercial and residential
human developments. Road construction and maintenance were almost equal. However,
in the case of Echinacea, the largest human influence on rarity in
certain taxa is undoubtedly wild-harvesting for the herb/dietary supplement
trade.
Echinacea harvesting is controversial and, likely due to mass commercialization
of the 1990s, it was particularly rampant throughout the Native American Indian
reservations across the Great Plains. In 1990, North Dakotan people were encouraged
to “just grab a shovel and start digging” while “environmentalists
in the state fear[ed] that gold fever [was] spreading among shovel wielding
collectors with dollar signs in their eyes.”36 “Rooting,”
as the digging of E. pallida var. angustifolia roots was called,
has been an economic opportunity for both native and non-native people, as
well as an ethical wildcrafting nightmare—where poachers have been known
to effectively clear out wild populations of the plants without concern for
preservation of the resources or the ecosystems.37
Early regulation of the rampant wild harvesting came in the form of tribal
resolutions in several states and later as legislation. For example, by 1999
North Dakota began to fine poachers $10,000 along with confiscation of their
vehicles.37 In Montana, a 3-year moratorium on the harvest of E.
angustifolia from state lands, pushed by herbalist R. Klein,37
led to the current Montana Code, which prohibits wild harvesting of E.
pallida var. angustifolia from public lands without a permit,
with a fine up to $1000 or 6 months in jail.38 Despite these
efforts, permits are still issued to dig for personal use (not commercial
sale) of E. pallida var. angustifolia roots in Montana, and
the harvesting regulation does not apply to aboriginal reservations or private
landowners. Clearly, Echinacea prairie varieties at risk from ecophysiological
factors and issues of genetic constraints, as discussed previously, have also
been under risk of human mismanagement.
Public land conservation is achieved through scientific input provided to
land management agencies and lobbying for federal legislation.39
Conservation and restoration on private lands was traditionally achieved through
zoning, condemnation, and tax regulation, with little success. Innovative
bottom-up approaches are increasingly addressing private land degradation
and slowing or halting the ravaging of natural ecological communities. Land
trusts, open-space tax incentives, “community-based conservation,”
and more have begun to effectuate stewardship. The Plant Conservation Alliance,
which formed its “Medicinal Plant Working Group” in 1999, has
established links between the Nature Conservancy and other institutions, NGOs,
and the public in a “bottom up” effort to change wild harvesting
practices. In fact, E. pallida var. pallida and E. pallida
var. angustifolia were cited on the list of “Medicinal Plant
Species in U.S. Commerce” as top priority warranting further study;
the authors based their assessment on 1989-1999 data for trade demand increases,
wild population declines, and species decline.40 Finally, government
grants ($3 US million in 2004) are now being awarded to protect plant species
on tribal lands.41
Take-Home Messages
Echinacea forbs compete poorly to fairly with native grasses in prairie
sites, resulting in low recruitment of Echinacea by seed. Significant
factors include the following:
Public lands are relatively easy to protect with scientific evidence (data
exist).
Private lands require new model for community-based natural resource management.
Habitat degradation due to development and human activity is the primary
cause of rarity.
Echinacea wildcrafting is not sustainable.
Conclusion
During the herbal renaissance of the 1990s, Echinacea plants were
the subject of much interest, common usage, and research scrutiny. Now, as
science and markets for medicinal plants continue to evolve in the 21st century,
the integration of findings from state-of-the-art original morphological,
molecular, and phytochemical work by the authors of this article, along with
ecological reports and the historical and regulatory literature of the times,
suggests that these North American native plants deserve their status as protected
resources. Moreover, it provides a comprehensive perspective into the biological
and political origins of Echinacea materials sourced as phytomedicines,
which is long overdue considering the focus on clinical evidence for Echinacea
health products and dietary supplements.
Acknowledgements
This manuscript benefited from comments by Dr. E. Small, Agriculture &
Agri-Food Canada, Eastern Cereal and Oilseed Research Centre, Ottawa. The
financial support of Trout Lake Farms LLC (Washington, USA), Amway/Nutralite
Corporation (USA), and MediPlant Consulting Services (Dennis V.C. Awang, PhD,
FCIC) of White Rock, BC, Canada, is gratefully acknowledged. We thank S. Mechanda
and J. Livesey (Research Technicians, Agriculture and Agri-Food Canada and
the University of Ottawa, Canada) for their contribution to the experimental
work.
Bernard R. Baum. Agriculture & Agri-Food Canada, Eastern Cereal and
Oilseed Research Center, Neatby Building, 960 Carling Avenue, Ottawa, Ontario,
Canada, K1A0C6. Corresponding author: e-mail: baumbr@agr.gc.ca;
phone: 613-759-1821.
Shannon E. Binns. University of British Columbia, Faculty
of Land and Food Systems, 2357 Main Mall, Vancouver, British Columbia, Canada,
V6T 1Z4.
John T. Arnason. Department of Biology, University of Ottawa, 30 Marie
Curie, Ottawa, ON, Canada K1N 6N5.
References
1. Cronquist A. Notes on the Compositae of the Northeastern United States
II. Heliantheae and Helenieae. Rhodora. 1945;47:396-398.
2. McGregor RL. The taxonomy of the genus Echinacea (Compositae).
Univ Kansas Sci Bull. 1968;48:113-142.
3. Bauer R, Wagner H. Echinacea species as potential immunostimulatory
drugs. In: Wagner H, Farnsworth N, eds. Economic and Medical Plant Research.
5th ed. London: Academic Press;1991:253-321.
4. Binns SE, Baum BR, Arnason JT. A taxonomic revision of the genus Echinacea
(Heliantheae; Asteraceae). Syst Bot. 2002;27:610-632.
5. Binns SE, Baum BR, Arnason JT. Phytochemical variation in populations
of Echinacea angustifolia (Asteraceae). Biochem Syst Ecol. 2002;30:837-854.
6. Binns SE, Livesey JF, Arnason JT, Baum BR. Phytochemical variation in
Echinacea Moench (Heliantheae: Asteraceae) from roots and flowerheads
of wild and cultivated populations. Jour Agric Food Chem. 2002;50:3673-3687.
7. Gower JC. A general coefficient of similarity and some of its properties.
Biometrics. 1971 27:857-871.
8. Binns SE, Baum BR, Arnason JT. Typification of Echinacea purpurea
(L.) Moench (Heliantheae: Asteraceae) and its implications on the correct
naming of two Echinacea taxa. Taxon. 2001;50:1169-1175.
9. McKeown KA. A Review of the taxonomy of the Genus Echinacea. In:
Janick J, ed. Perspectives on New Crops and New Uses. Alexandria, VA:
ASHS Press; 1999:482-489.
10. Binns SE, Baum BR, Arnason JT. Taxon. Proposal to conserve the
name Rudbeckia purpurea L. (Asteraceae) with a different type. 2001;50:1199-1200.
11. Hitchcock CL, Cronquist A, Ownbey M, Thompson JW. Vascular plants
of the Pacific Northwest. Vol 5. Seattle: University of Washington Press.
1955-69.
12. Radford AE, Hardin JW, Massey JR, Core EL, Radford LS, eds. Vascular
flora of the southeastern United States. Vol 1. Chapel Hill: University
of North Carolina Press; 1980.
13. Urbatsch LE, Jansen RK. Phylogenetic affinities among and within the
coneflower genera (Asteraceae, Heliantheae), a chloroplast DNA analysis.
Syst Bot. 1995;20:28-39.
14. Robinson H. Studies in the Heliantheae (Asteraceae), XIV: Validation
of subtribes. Phytologia. 1978;41:39-44.
15. Robinson H. A revision of the tribal and subtribal limits of the Heliantheae
(Asteraceae) Smithsonian Contrib Bot. 1981;51:1-102.
16. Urbatsch LE, Baldwin BG, Donoghue MJ. Phylogeny of the coneflowers and
relatives (Heliantheae: Asteraceae) based on nuclear rDNA internal transcribed
spacer (ITS) sequences and chloroplast DNA restriction site data. Syst
Bot. 2000;25:539-565.
17. Mechanda SM, Baum BR, Johnson DA, Arnason JT. Analysis of diversity
of natural populations and commercial lines of Echinacea using AFLP. Can
J Bot. 2004;82:461-484.
18. US Fish and Wildlife Service. Threatened and endangered Species System—E.
laevigata and E. tennesseensis. List updated March 31, 2004. Available
at: http://ecos.fws.gov/species_profile/SpeciesProfile?spcode=Q293
and http://ecos.fws.gov/species_profile/SpeciesProfile?spcode=Q1VU.
19. Kim DH, Heber D, Still DW. Genetic diversity of Echinacea species based
upon amplified fragment length polymorphism markers. Genome. 2004;47:102-111.
20. Clark AG, Lanigan CMS. Prospects for estimating nucleotide divergence
with RAPDs. Mol Biol Evol. 1993;10:1096-1111.
21. Kapteyn J, Goldsbrough PB, Simon JE. Genetic relationships and diversity
of commercially relevant Echinacea species. Theor Appl Genet.
2002;105:369-376.
22. Baum BR, Mechanda S, Livesey JF, Binns SE, Arnason JT. Predicting quantitative
phytochemical markers in single Echinacea plants or clones from their DNA
fingerprints. Phytochemistry. 2001;56:543-549.
23. Bauer R. Echinacea: Biological effects and active principles.
In: Lawson LD, Bauer R, eds. Phytomedicines of Europe: Chemistry
and Biological Activity. ACS symposium series 691. Washington, DC: American
Chemical Society;1998:140-157.
24. Bauer R, Khan I, Wagner H. TLC and HPLC analysis of Echinacea pallida
and E. angustifolia roots. Planta Med. 1988;54:426-430.
25. Binns SE, Inparajah I, Baum BR, Arnason JT. MeJ increases alkamide and
ket
|