|
|
|
|
|
|
|
|
|
Issue: 82 Page: 34-41
The Expanding Market and Regulatory Challenges of Supplements for Pets in the United States
by Courtney Cavaliere
HerbalGram. 2009;82:34-41 American Botanical Council
By Courtney Cavaliere
Many men and women know that they can take natural remedies such as echinacea (Echinacea spp., Asteraceae) to help ward off colds or valerian (Valeriana officinalis, Valerianaceae) to combat anxiety. In increasing numbers, pet owners have begun to realize that similar natural supplements are also available to treat their sneezing Labradors or to calm their high-strung tabbies. Recent market statistics indicate that sales of supplements for companion animals* are on the rise. Tinctures, tablets, and powders designed to supplement the diets and treat the ailments of humankind’s furry friends are increasingly available, despite on-going regulatory challenges. These products are expected to experience continued growth in the near future, indicating that the supplement industry may quickly be “going to the dogs.”
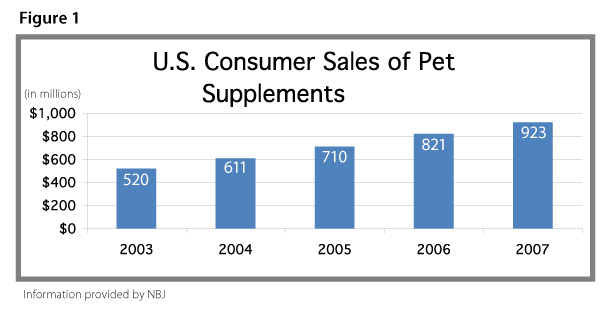
The Pet Supplement Market Nutrition Business Journal (NBJ) has estimated that US pet supplement sales reached $923 million in 2007, an increase of 8% over 2006 sales (See Figure 1).1 NBJ found that the overall natural pet and pet nutrition market (defined as pet supplements, natural and organic pet foods, and pet supplies and other products) exceeded $2.1 billion in 2007, of which supplements accounted for 43% of sales.
Research published recently by Packaged Facts, meanwhile, estimated US retail sales of pet supplements and nutraceutical treats at $1.2 billion in 2007, with 74% of this figure representing supplement sales.2 According to the Packaged Facts report, the majority of pet supplements (51%) are purchased for horses, while dog supplements represent 38% of the market and cat supplements represent 6%. The company Native Remedies (Boca Raton, FL), which has manufactured natural supplements and treatments for men and women since its founding in 2002, launched a line of supplements for dogs and cats under the brand name PetAlive® in 2005. PetAlive products are compound formulas consisting of herbal and other natural ingredients, as well as a range of homeopathic remedies. According to George Luntz, president and co-founder of Native Remedies, sales of PetAlive products have more than tripled over the past few years. “The growth rate has pretty much surpassed the rate of our human products—which are also rising,” said Luntz (oral communication, February 19, 2009). The company initiated a line of products for horses in March of 2009. Many supplements for pets are based on or use herbal ingredients. According to William Bookout, president of the National Animal Supplement Council (NASC), probably 15% of the approximately 125 pet supplement companies that belong to NASC produce herb-specific supplements for dogs, cats, and horses (oral communication, November 10, 2008). He added that around 80% of NASC member companies probably use herbs as ingredients in some of their product formulas. Greg Tilford, president of Animal Essentials (Victor, MT), stated that herbal supplements for animals have definitely become a fast-growing market niche. Animal Essentials established a line of herbal medicinal pet products under the brand name Animals’ Apawthecary in 1995, and the company now produces over 40 single-herb extracts in a glycerin or mild alcohol base and over 15 combination formulas in liquids as botanical supplements for pets. According to Tilford, Animal Essentials’ product line has experienced double-digit growth almost every year since its inception (G. Tilford, oral communication, October 28, 2008). The latest annual meeting of the American Holistic Veterinary Medical Association (AHVMA), held in October of 2008, also gave an indication that there is growing availability of herbal veterinary products. According to Nancy Scanlan, DVM, president of the Veterinary Botanical Medical Association (VBMA) and a veterinarian at the Sherman Oaks Veterinary Group in California, about one-third of the exhibitors at the meeting were herbalists (oral communication, October 27, 2008). Dr. Scanlan added that the rise in associations supporting complementary and alternative medicine techniques for animals reinforces growth in the field of alternative healthcare for pets. Over the past 2 decades, the number of associations supporting the use of acupuncture for animals has increased from 1 to 3. The VBMA, an education-based organization dedicated to promoting the responsible use of herbal medicine for animals, was founded in 2002. Many factors have been fueling growth in the pet supplement industry. Pet ownership, for instance, is on the rise.1 According to the 2007-2008 National Pet Owners Survey, 63% of US households own a pet, whereas 56% of households owned a pet when the survey was first conducted in 1988.3 Luntz noted that more and more people throughout the world have begun to take an interest in using natural treatment options to care for their own health, as well as the health of their families. “Pets are pretty much part of our families,” he explained. “It makes sense that people would want to include them in their quest for healthier living.” “There is now very strong awareness that the food supply is not what it used to be and that there is a need for people to supplement their diets. That carries over for animals, as well,” said Tom Cameron, DVM, veterinary technical support for Standard Process Inc. (Palmyra, WI), a company that manufactures nutritional whole food supplements for both humans and animals (oral communication, February 16, 2009). “A lot of health conditions are caused by nutritional deficiencies, and foods for animals, in particular, are highly processed.” Use of supplements for animals is also rising due to the growing recognition of their effectiveness and gentleness, particularly in comparison to some pharmaceuticals. “In my practice, almost every animal I see goes out the door with some herbs,” said Dr. Scanlan. “I use herbs both because of situations where they are more effective than Western medicine and situations where they have less side effects than Western medicine.” Dr. Scanlan noted that, for instance, there are no conventional pharmaceutical medications that are particularly effective for treating prostate problems in animals, and herbal supplements are therefore a good choice for veterinarians confronted with this condition. She has found that saw palmetto (Serenoa repens, Arecaceae), either alone or with Chinese herbal formulas, often helps where Western medicine is less effective. She also frequently recommends herbal treatments for animals with arthritis, since herbal medications do not have the adverse effects on liver and kidneys that are often associated with pharmaceuticals.
Regulation of Supplements for Pets
Supplements for pets have been able to achieve impressive strides in the marketplace despite a complicated legal status and a lack of formal regulations.
The Dietary Supplement Health and Education Act of 1994 (DSHEA) established regulations for human dietary supplements at a time when there were few similar products for pets on the market.4 DSHEA did not specifically address the topic of supplements for pets, but a posting in the Federal Register later specified that the US Food and Drug Administration (FDA) does not consider such supplements to be covered under DSHEA.5 Supplements marketed for dogs, cats, and horses have therefore been left with 2 possible legal categories under US law—they may be defined as animal foods/feeds or animal drugs.4,6 Most supplement products for pets are classified by the manufacturers as nutritional or feed supplements. However, hundreds of ingredients commonly used for human and pet dietary supplements are not technically approved for use in animal feed products. The other regulatory option for pet supplements, as animal drugs, requires that supplement marketers submit a New Animal Drug Application to the FDA’s Center for Veterinary Medicine (CVM), demonstrating the product’s safety and efficacy according to certain criteria. According to Book-out, these applications, and the testing that they require, are expensive and problematic. In particular, companies cannot recover the considerable costs incurred through product development and testing, since, in most cases, natural substances cannot be patented. Many animal supplements are thus discouraged from even attempting to meet the criteria for “drug approval.” Most supplements for animals are therefore considered animal feeds that contain unapproved ingredients or unapproved drugs, depending on intended use.7 Supplements for animals were nearly removed from the marketplace beginning in 2002, after the American Association of Feed Control Officials (AAFCO) organized a committee to develop an enforcement strategy for unrecognized, undefined animal feed ingredients, as well as accepted ingredients that were being marketed for unapproved uses.† The NASC (www.nasc.cc), however, which was established in 2002, was able to placate concerned consumers and regulators by initiating effective self-regulation measures for the pet supplement industry. The NASC, a nonprofit dedicated to providing a unified voice for animal health and nutritional supplement companies, immediately created quality control guidelines and instituted risk monitoring procedures for the industry. Member companies of the NASC must use a written quality control manual, follow proper labeling guidelines, and implement an adverse event reporting system for tracking complaints and safety concerns.8 All NASC members must demonstrate their compliance with NASC protocols by having completed or scheduled an independent facility audit. Those companies that have successfully completed an audit are able to use the NASC Quality Seal on their products, Web sites, and advertisements, as a symbol to the public that the company conforms to strict quality standards. About 90% of companies that produce supplements for pets are members of NASC.1 Animal Essentials is a member of NASC and conforms to the organization’s quality protocols. The company uses only human-grade ingredients, identifies all ingredients using at least US Pharmacopeia standards, and tests all materials for marker compounds, microbes, and shelf-life. “I think it’s safe to say that my company has higher standards than some companies selling similar products for humans,” said Tilford.
Standard Process is also a member of NASC and adheres to very high quality control standards. Standard Process tests products and ingredients multiple times throughout the manufacturing process in its onsite laboratory and participates in routine inspections by the FDA and regulating agencies. Dr. Cameron added that the ingredients used in the Standard Process Veterinary Formulas product line are also used in the company’s line of human products, and all of the products are developed in the same manufacturing facility. Both sets of products are therefore produced according to the same good manufacturing practices (GMPs) and high quality control standards.
Although not a member of NASC, Native Remedies performs rigorous testing on ingredients and produces both human and pet products according to the same GMPs and quality standards. The company also registers its manufacturers as approved food facilities and individually registers homeopathic medicines (including some PetAlive products) as over-the-counter products with the FDA.
Due to quality control and risk assessment efforts by the industry, the FDA-CVM currently regulates pet supplements with an intended use other than feed as unapproved drugs of low regulatory priority.7 However, Tilford stated that the industry will ultimately need more regulation. He argued that the pet supplement industry is currently in a situation similar to the one faced by the human dietary supplement industry before the passage of DSHEA. “It’s like being in the herbal industry 20 years ago,” said Tilford. “History is repeating itself.” Bookout also stated that more regulation is bound to be implemented within the pet supplement industry in the future, and he claimed that such regulation is not necessarily detrimental to pet supplement companies or to the NASC. “Anybody who is a responsible participant within an industry should not be opposed to regulation that is fair, reasonable, responsible, and consistent,” said Bookout. According to Bookout, the NASC has always been interested in achieving a viable long-term solution to the industry’s regulatory challenges. “We work very cooperatively and very constructively with regulatory authorities at the federal and state level,” said Bookout. He explained that the FDA and state officials have expressed interest in ultimately formulating sufficient and consistent regulations for pet supplements, and the NASC intends to assist in those efforts and help ensure that future regulations are fair and responsible. “If we are not proactive in supporting that objective, then it will be defined for us. It’s better to shape your own destiny than to have it defined for you,” he said. 
Twenty-one year-old gelding Rico was given a course of Standard Process’s veterinary formula Equine Metabolic Support. Before treatment, Rico was overweight, lame, had a fasting serum insulin of greater than 1600 units/mopl, and had fat pads—especially on the thorax and around his tail. After a month of treatment, he lost significant mass on his neck and the fat pads diminished, his fasting serum insulin dropped to 601 units, and he walked without evidence of pain. Equine Metabolic Support contains such ingredients as licorice (Glycyrrhiza glabra, Fabaceae) root, cinnamon (Cinnamomum verum, Lauraceae), and green tea (Camellia sinensis, Theaceae) extract. Photos © 2009 Standard Process.
Safety of Supplements for Pets
The actual and perceived safety of supplements for pets undoubtedly affects their sales in the marketplace. The FDA-CVM recently requested that the National Research Council of the National Academies investigate the safety of supplements for dogs, cats, and horses. The National Research Council convened a committee of experts to analyze this issue, and they released a report on the topic in 2008.9
The committee concluded that there is limited safety data for determining safe use of supplements for animals. The committee specifically assessed the safety of 3 supplements for dogs, cats, and horses—lutein (a carotenoid, usually derived from marigolds, Tagetes spp., Asteraceae), evening primrose (Oenothera biennis, Onagraceae) oil, and garlic (Allium sativum, Liliaceae). They discovered a lack of quality safety data on those supplements to determine safety in drugs and animal food additives, and they wrote that they could only report on historical safe intakes and estimate presumed safe intakes for the 3 animal supplements. The committee noted that supplements and ingredients considered safe in humans and other species are not always safe in horses, dogs, and cats. Excessive garlic, in particular, can cause hemolytic anemia in all 3 species. They recommended that clear and precise regulations for animal supplements be established, as well as a comprehensive adverse event reporting system that is not limited by membership or paid access and that generates accurate and reliable data. Bookout issued a response to this report, in which he pointed out that the majority of the pet supplement industry belongs to NASC and participates in adverse event reporting through the organization.10 NASC member companies report all adverse events that they have received to the NASC on a monthly basis, and the NASC has built alerts into its system so that it is notified anytime a serious adverse event is reported by a member company (W. Bookout, oral communication, November 10, 2008). The organization’s NASC Adverse Event Reporting System (NAERS) can organize and retrieve data regarding adverse events according to company, product, or ingredient. The NAERS system defines an adverse event broadly as any complaint for a product linked to a physical effect or health problem that may be (but is not necessarily) connected to or associated with use of the product—including transient events like vomiting, diarrhea, etc. According to the NAERS system, there have been, on average, 0.31 adverse events reported per million pet supplement administrations (dosage unit administered to an animal) sold by NASC member companies. NASC also collects information on serious adverse events, defined as any event with a transient incapacitating effect or a long-term or permanent health effect that requires follow-up with a veterinarian. According to the NAERS system, there have been, on average, 0.001 serious adverse events reported per million pet supplement administrations sold by NASC member companies. Bookout noted that the incidence of adverse events is very low, particularly in light of the organization’s broad definition of adverse events. He added that companies’ and NASC’s adverse event reporting systems are important for vigilant risk management and for identifying early warning signs of potential dangers. All NASC member companies also employ warning or cautionary statements in labeling for products that contain certain herbal ingredients, as recommended by the NASC Scientific Advisory Committee. For instance, supplements with garlic must include a cautionary statement in product labels advising consumers not to administer the product to a pet prior to surgery, since garlic can prolong bleeding and clotting time. Further, some herbal ingredients are banned from member companies’ products. Bookout explained that the FDA-CVM has recommended that kava (Piper methysticum, Piperaceae), comfrey (Symphytum spp., Boraginaceae), and pennyroyal (Mentha pulegium, Lamiaceae) oil not be used in pet food products, and the NASC has prohibited these herbal ingredients in supplements due to the agency’s recommendation. Kava has been associated with possible liver toxicity per some human case reports (although no causality has been confirmed), comfrey contains hepatotoxic pyrrolizidine alkaloids, and pennyroyal oil is thought to contain the toxin pulegone and has been linked to the death of one dog following dermal exposure according to a case report.11
Efficacy of Supplements for Pets
According to Dr. Scanlan, there has not been much research into the efficacy of herbal medicines for pets. However, she added that a research committee of the AHVMA has been providing grants for many research projects and encouraging researchers to test pet supplements for efficacy. Some supplement companies have begun partnering with veterinarians, university veterinary clinics, and animal shelters to test the effectiveness of their products.
A randomized, controlled clinical study to assess an herbal supplement for canine pain and lameness was initiated in October 2008 at Colorado State University.12 In this study, 36 dogs with confirmed osteoarthritis will be given either the herbal supplement Pet Relief® or a placebo over a 5week period in order to test the supplement’s effectiveness and to investigate any complications associated with its use. Pet Relief, manufactured by RZN Nutraceuticals (Orange Park, FL), contains juniper (Juniperus communis, Cupressaceae) berry, goldenrod (Solidago virgaurea, Asteraceae), dandelion (Taraxacum officinale, Asteraceae) leaf, meadowsweet (Filipendula ulmaria, Rosaceae) herb, willow (Salix alba, Salicaceae) bark, and cranberry (Vaccinium macrocarpon, Ericaceae). “Having a clinical study done by an independent third party and with the collection of objective measurements of improvement in study subjects puts RZN at the leading edge of this type of research for animal products,” said Mark Lubin, founder of RZN (e-mail, November 18, 2008). “We wanted to be able to prove by direct measurement that the results obtained were not imaginary or subjective.” According to Narda Robinson, DVM, director of Colorado State University’s Center for Comparative and Integrative Pain Medicine and lead investigator of the study: “It is a rare company that will support a randomized, controlled, blinded study. I hope that, with this study, we can encourage more herbal manufacturers to clinically test their products” (e-mail, October 21, 2008). “I am concerned about the health and safety of animals treated with herbs for which neither the mechanism of action, nor the purity of the product, nor the safety in small animals is known,” she continued. “Although many herb courses teach veterinarians how to prescribe Chinese herbs, they do so, all too often, from a metaphorical, i.e., Traditional Chinese Medicine perspective. For me, saying a product will ‘eliminate wind’ or ‘remove dampness’ is insufficient. That may have been okay centuries or millennia ago, but as bio-medically based, scientifically trained healthcare professionals, we must go beyond the metaphors and insist on mechanisms. We should expect medical professionals to know how the herbal ingredients in a product work, what the potential adverse effects are, and how the herbs may interact with other herbs or medications. My primary concern is helping animals and keeping them safe.” Dr. Cameron explained that Standard Process has tested some of its veterinary products in various university veterinary schools, and the company is negotiating with other schools to arrange future studies. The company has also tested its equine formulas through local veterinarians in southern Wisconsin. In February of 2009, Standard Process initiated a study of its product Feline Immune System SupportTM, a combination product containing such herbal ingredients as eleuthero (Eleutherococcus senticosus, Araliaceae) and Indian gooseberry (Emblica officinalis, Euphorbiaceae), through a cat shelter in Ohio. According to Dr. Cameron, 75% of cats admitted to the shelter in 2008 had to be treated at some point during their stay with antibiotics for upper respiratory tract infections—a common problem in shelters. All cats that enter the shelter are now being given Feline Immune System Support on a preventative basis, to see if this will lower the incidence of such infections among the shelter’s cats. If preliminary results from this and other studies are promising, then the company plans to perform more rigorous clinical trials with monitored samples and biomarkers. Greg Tilford said that Animal Essentials does not test the efficacy of its products through clinical trials. However, the company works with between 400 and 450 veterinarian clients in the development and production of botanical pet supplements, and he said that the successful use of Animals’ Apawthecary products by veterinarians attests to their safety and efficacy. “The proof is in the pudding,” said Tilford. Similarly, Native Remedies does not conduct clinical trials on its products for pets. According to Luntz, natural ingredients do not typically raise safety concerns the way that pharmaceutical agents do, and the natural ingredients used in the PetAlive products are backed by clinical research and a history of hundreds of years of traditional use. “We don’t choose our ingredients lightly,” said Luntz. “They have to have strong research supporting them, as well as a long history of safe usage and efficacy.”
Different Forms of Pet Supplementation
Although the market for supplements for pets appears to be growing, it is important to note that use of supplements for animals may extend beyond pet-specific products. Men and women sometimes use supplements intended for humans to treat pets, as well.
Luntz noted that Native Remedies initiated the PetAlive line of products after receiving numerous inquiries from the company’s consumer base for pet products. The company initially guided its customers on how to use Native Remedies products for humans in different dosages to treat their pets, but “with as much demand as we were seeing, we decided to launch a product line for pets in 2005,” said Luntz. Standard Process’s human supplements were likewise recommended for pets with nutritional needs, prior to the establishment of the company’s veterinary line. The company created its veterinary line in 2002, based on the idea that animals have different nutritional needs than humans. Dr. Cameron noted that pet-specific formulations may be the preferred treatment option for that reason. The company RZN manufactures supplements for both people and pets, and the differences between Pet Relief and the versions of that product for humans (Arthi-Zen Relief®) and horses (Horse Relief®) illustrate how care may need to be taken in administering particular herbs and supplements to certain species. According to Lubin, dogs seem more sensitive to specialized dosing requirements, so Pet Relief provides more dosage recommendations for weight classes than the other 2 products. Further, for Pet Relief, cranberry extract has been substituted for the whole grape (Vitis vinifera, Vitaceae) extract found in Arthi-Zen Relief and Horse Relief. “We made this substitution because research is available which shows that grape and its derivatives may be harmful to the excretory systems of canines, whereas cranberry is not,” said Lubin.
The Future of the Industry
NBJ has forecasted continued strong growth of the natural pet and pet nutrition market, of which supplements for animals are a key category. NBJ noted, however, that shifts are likely to occur in the types of supplements sold and the outlets through which supplements are sold.1 Packaged Facts likewise has estimated that the industry of pet supplements and nutraceutical treats will experience continued growth. Caring for aging pet populations could generate particular market interest in the coming years.2
According to Dr. Scanlan, there is a need for more proof, more case studies, and more scientific evidence to support the growing field of botanical supplements for pets. She recommends that new-comers to the field collaborate with the VBMA and NASC as much as possible, and that they invest in efforts to educate veterinarians and consumers regarding the products they develop. “The more help I get from an herb company, the more likely I am to use their products,” she added.
†Many herbal ingredients have been given GRAS status by the FDA and AAFCO and are categorized as feed additives, with their intended use defined as “spices and other natural seasonings and flavorings” or “essential oils, oleoresins, and natural extractives.” When such ingredients are incorporated into an animal supplement with the purpose being to “effect the structure or any function of the body,” they are considered by regulatory agencies to be unapproved ingredients, thereby causing the product to be considered “adulterated.”
References
- Joint, omega-3 and other supplements prove popular for Bowser and Fluffy. Nutrition Business Journal. June 2008.
- Pet supplements and nutraceutical treats in the US. Packaged Facts. February 2008.
- Industry Statistics & Trends. American Pet Products Manufacturers Association Web site. Available at: http://americanpetproducts.org/press_industrytrends.asp. Accessed November 18, 2008.
- Regulation of Animal Health Supplements—A Historical Summary. National Animal Supplement Council Web site. Available at: http://nasc.cc/index. php?option=com_content&task=view&id=19&Itemid=41. Accessed November 10, 2008.
- US Food and Drug Administration. Inapplicability of the Dietary Supplement Health and Education Act to Animal Products. 61 Federal Register 78. April 22, 1996. Available at: http://bulk.resource.org/gpo.gov/register/1996/1996_17706.pdf. Accessed November 14, 2008.
- Bookout W. Regulatory overview of “dietary supplements” for animals: things you need to know and mistakes not to make. Powerpoint presentation given at SupplySide West; October 23, 2008; Las Vegas, NV.
- Bookout W, Khachatoorian LB. Regulation and quality control. In: Wynn SG, Fougere BJ, eds. Veterinary Herbal Medicine. St. Louis, MO: Mosby Elsevier; 2007:99-120.
- The NASC Seal. National Animal Supplement Council Web site. Available at: http://nasc.cc/index.php?option=com_content&task=view&id=40&Itemid=80. Accessed November 7, 2008.
- Safety of Dietary Supplements for Horses, Dogs, and Cats: Report in Brief. The National Academies; 2008. Available at: http://dels.nas.edu/dels/rpt_briefs/satety_ of_dietary_supplements_final.pdf. Accessed November 7, 2008.
- Bookout, B. Report from the National Research Council on the safety of dietary supplements for horses, dogs, and cats—comments from the NASC president. National Animal Supplement Council Newsletter—Supplemental. November 2008.
- Poppenga RH. Herbal medicine: potential for intoxication and interactions with conventional drugs. In: Wynn SG, Fougere BJ, eds. Veterinary Herbal Medicine. St. Louis, MO: Mosby Elsevier; 2007:183-207.
- Clinical study at Colorado State University looks at herbal supplements to address canine pain and lameness [press release]. Fort Collins: Colorado State University; October 14, 2008.
|
|
|
|
|
|
|